Methanesulfonamide

Methanesulfonamide structure
|
Common Name | Methanesulfonamide | ||
|---|---|---|---|---|
| CAS Number | 3144-09-0 | Molecular Weight | 95.12 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 208.2±23.0 °C at 760 mmHg | |
| Molecular Formula | CH5NO2S | Melting Point | 85-89 °C(lit.) | |
| MSDS | USA | Flash Point | 79.7±22.6 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of MethanesulfonamideMethanesulfonamide is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | Methanesulfonamide |
|---|---|
| Synonym | More Synonyms |
| Description | Methanesulfonamide is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog | |
| In Vitro | 甲磺酰胺与亚硫酰氯、氰酸盐、二硫化碳及酮、醛反应制备药物、光亮剂等目标分子。它还用于生物学研究,以预测蛋白质配体复合物的结合亲和力和结合模式。 |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 208.2±23.0 °C at 760 mmHg |
| Melting Point | 85-89 °C(lit.) |
| Molecular Formula | CH5NO2S |
| Molecular Weight | 95.12 |
| Flash Point | 79.7±22.6 °C |
| Exact Mass | 95.004097 |
| PSA | 68.54000 |
| LogP | -1.29 |
| Vapour Pressure | 0.2±0.4 mmHg at 25°C |
| Index of Refraction | 1.460 |
| InChIKey | HNQIVZYLYMDVSB-UHFFFAOYSA-N |
| SMILES | CS(N)(=O)=O |
| Storage condition | 2~8℃ |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 29350090 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |
|
Anti-inflammatory effects of N-acylethanolamines in rheumatoid arthritis synovial cells are mediated by TRPV1 and TRPA1 in a COX-2 dependent manner.
Arthritis. Res. Ther. 17 , 321, (2015) The endocannabinoid system modulates function of immune cells and mesenchymal cells such as fibroblasts, which contribute to cartilage destruction in rheumatoid arthritis (RA). The aim of the study wa... |
|
|
Inhibition of cyclooxygenase-2 causes a decrease in coronary flow in diabetic mice. The possible role of PGE2 and dysfunctional vasodilation mediated by prostacyclin receptor.
J. Physiol. Biochem. 71 , 351-8, (2015) Several lines of evidence suggest that cyclooxygenase-2 (COX-2) activity can have a beneficial role in the maintenance of vascular tone of the blood vessels in diabetes. Specifically, the increased pr... |
|
|
In vitro antitumor mechanism of (E)-N-(2-methoxy-5-(((2,4,6-trimethoxystyryl)sulfonyl)methyl)pyridin-3-yl)methanesulfonamide.
Mol. Pharmacol. 87(1) , 18-30, (2015) ON01910.Na [sodium (E)-2-(2-methoxy-5-((2,4,6-trimethoxystyrylsulfonyl)methyl)phenylamino)acetate; Rigosertib, Estybon], a styryl benzylsulfone, is a phase III stage anticancer agent. This non-ATP com... |
| MFCD00007940 |
| aminosulfane dioxide |
| Methanesulfonamide |
| Methylsulfonamide |
| methansulfonamid |
| methanesulfonyl amine |
| Methane sulfonamide |
| EINECS 221-553-6 |
| methylsulfonylamine |
| Methanesulphonamide |
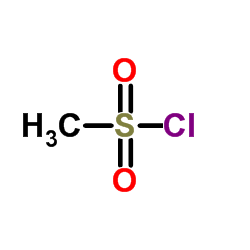 CAS#:124-63-0
CAS#:124-63-0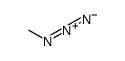 CAS#:624-90-8
CAS#:624-90-8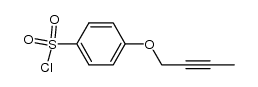 CAS#:286459-94-7
CAS#:286459-94-7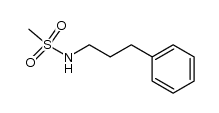 CAS#:362665-07-4
CAS#:362665-07-4 CAS#:80653-53-8
CAS#:80653-53-8![Methyl 2-[[(4-fluorophenyl)sulfonyl]amino]3-methyl benzoate Structure](https://image.chemsrc.com/caspic/463/357616-23-0.png) CAS#:357616-23-0
CAS#:357616-23-0 CAS#:74-88-4
CAS#:74-88-4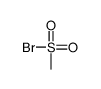 CAS#:41138-92-5
CAS#:41138-92-5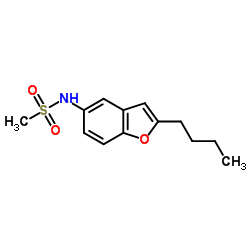 CAS#:437652-07-8
CAS#:437652-07-8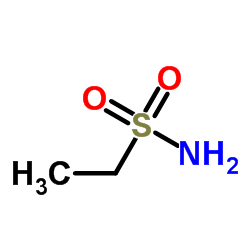 CAS#:1520-70-3
CAS#:1520-70-3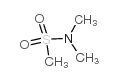 CAS#:918-05-8
CAS#:918-05-8 CAS#:202658-88-6
CAS#:202658-88-6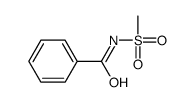 CAS#:22354-11-6
CAS#:22354-11-6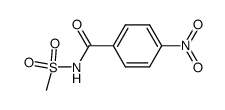 CAS#:53864-63-4
CAS#:53864-63-4 CAS#:22457-04-1
CAS#:22457-04-1 CAS#:2374-61-0
CAS#:2374-61-0 CAS#:23705-43-3
CAS#:23705-43-3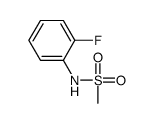 CAS#:98611-90-6
CAS#:98611-90-6
