|
~63% |
|
~6% |
|
~25% |
|
~7% |
|
~6% |
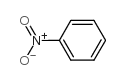

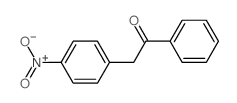

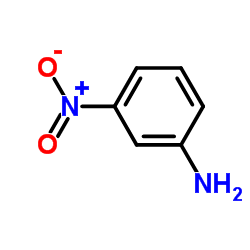
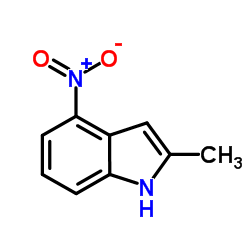
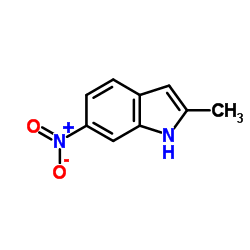
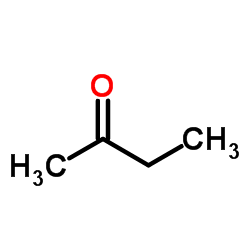

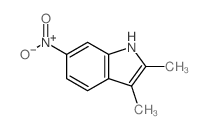
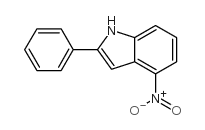
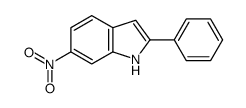
![N-(3-nitrophenyl)-N-[(E)-1-phenylethylidene]amine Structure](https://image.chemsrc.com/caspic/335/663177-71-7.png)