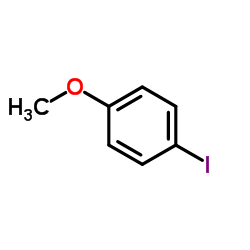
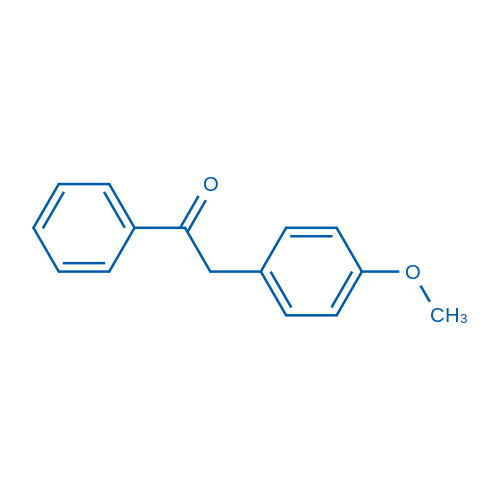
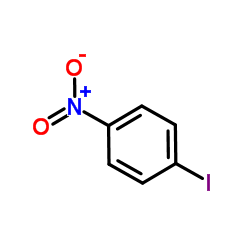
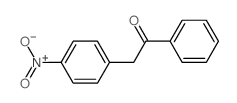
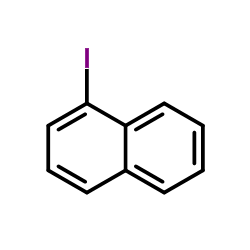
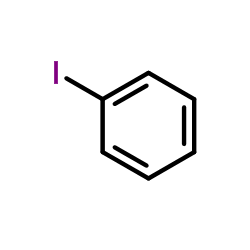
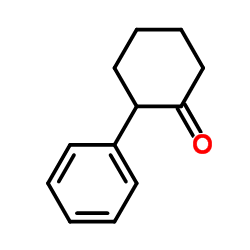
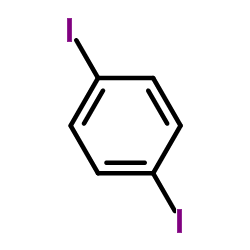
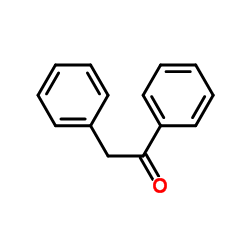
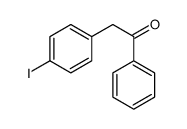
|
~55% |
|
~18% |
|
~59% |
|
~10% |
|
~35% |