丙烯酸
一般危化品

丙烯酸结构式
|
常用名 | 丙烯酸 | 英文名 | Acrylic acid |
|---|---|---|---|---|
| CAS号 | 79-10-7 | 分子量 | 72.063 | |
| 密度 | 1.1±0.1 g/cm3 | 沸点 | 141.0±0.0 °C at 760 mmHg | |
| 分子式 | C3H4O2 | 熔点 | 13 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 54.4±0.0 °C | |
| 符号 |




GHS02, GHS05, GHS07, GHS09 |
信号词 | Danger |
丙烯酸用途【用途一】 通过均聚或共聚制备高聚物,用于涂料、粘合剂、固体树脂、模塑料等 【用途二】
丙烯酸及其系列产品,主要是其酯类,近年得到迅速发展。如象乙烯、丙烯、氯乙烯、丙烯腈等那样,发展成为重要的高分子化学工业的原料。丙烯酸及其酯类作为高分子化合物的单体,世界总产量已超过百万t,而由其制成的聚合物和共聚物(主要是乳液型树脂)的产量将近500万t。这些树脂的应用遍及涂料,塑料、纺织、皮革、造纸、建材,以及包装材料等众多部门。丙烯酸及其酯类可供有机合成和高分子合成,而绝大多数是用于后者,并且更多地是与其他单体,如乙酸乙烯、苯乙烯、甲基丙烯酸甲酯等进行共聚,制得各种性能的合成树酯、功能高分子材料和各种助剂等。主要应用领域:(1)经纱上浆料 由丙烯酸、丙烯酸甲酯、丙烯酸乙酯、丙烯腈、聚丙烯酸铵等原料配制的经纱上浆料,比聚乙烯醇上浆料容量退浆,节省淀粉。(2)胶粘剂 用丙烯酸、丙烯酸甲酯、丙烯酸乙酯、丙烯酸-2-乙基己酯等共聚乳胶,可作静电植绒、植毛的胶粘剂,其坚牢性和手感好。(3)水稠化剂 用丙稀酸和丙烯酸乙酯共聚物制成高分子量的粉末。可作稠化剂,用于油田,每吨产品可增产500t原油,对老井采油效果较好。(4)铜版纸涂饰剂 用丙烯酸、丙烯酸丁酯、丙烯酸-2-乙基己酯、苯乙烯等四元共聚乳胶作铜版纸涂料,保色不泛黄,印刷性能好,不粘辊,比丁苯胶乳好可节省干酷素。(5)聚丙烯酸盐类 利用丙烯酸可生产各种聚丙烯酸盐类产品(如铵盐、钠盐、钾盐、铝盐、镍盐等)。用作凝集剂、水质处理剂、分散剂、增稠剂、食品保鲜剂耐酸碱干燥剂,软化剂等各种高分子助剂。 【用途三】 1.高吸水性树脂 2.高分子聚合的官能单体 3.有机合成4.高分子电解质 更多
|
| 中文名 | 丙烯酸 |
|---|---|
| 英文名 | acrylic acid |
| 中文别名 | 败酯酸 | 败脂酸 | 2-丙烯酸 | 乙烯基甲酸 | 丙烯酸(HPAA) |
| 英文别名 | 更多 |
| 密度 | 1.1±0.1 g/cm3 |
|---|---|
| 沸点 | 141.0±0.0 °C at 760 mmHg |
| 熔点 | 13 °C(lit.) |
| 分子式 | C3H4O2 |
| 分子量 | 72.063 |
| 闪点 | 54.4±0.0 °C |
| 精确质量 | 72.021126 |
| PSA | 37.30000 |
| LogP | 0.28 |
| 外观性状 | 无色液体 |
| 蒸汽密度 | 2.5 (vs air) |
| 蒸汽压 | 3.4±0.5 mmHg at 25°C |
| 折射率 | 1.422 |
| 储存条件 | 1.密封阴凉避光保存。远离火种、热源,防水、防潮、防冻。贮存温度最好在15-20℃之间。为防止贮存时冻结,一般使其水溶液含量保持在80%(凝固点-5.5℃)或用溶剂稀释。 2.采用聚乙烯衬里的铁桶包装,每桶重200kg,也可用不锈钢或碳钢贮槽,但必须防止水分和湿气,以防生锈造成丙烯酸聚合。在贮存和运输时都要添加200*10-6的阻聚剂。按“腐蚀性化学品规定”贮运。 3.储存注意事项通常商品加有阻聚剂。储存于阴凉、通风的库房。远离火种、热源。库温不宜超过5℃(装于受压容器中例外)。包装要求密封,不可与空气接触。应与氧化剂、碱类分开存放,切忌混储。不宜大量储存或久存。采用防爆型照明、通风设施。禁止使用易产生火花的机械设备和工具。储区应备有泄漏应急处理设备和合适的收容材料。 |
| 稳定性 | 1.化学性质活泼,遇光、热、过氧化物等容易发生聚合。有较强的腐蚀性,易燃。受热易分解产生有毒气体。具有双键及羧基官能团的联合反应,可发生加成反应、官能团反应以及酯交换反应。常用以制备多环和杂环化合物。易被氢还原成丙酸,遇碱能分解成甲酸和乙酸。 2.本品有较强的腐蚀性,中等毒性。其水溶液或高浓度蒸气会刺激皮肤和黏膜。大鼠口服LD50为590mg/kg。注意不得与丙烯酸溶液或蒸汽接触,操作时要佩戴好工作服和工作帽、防护眼镜和胶皮手套。生产设备应密闭。工作和贮存场所要具有良好的通风条件。 3.稳定性 稳定 4.禁配物 强氧化剂、强碱 5.避免接触的条件 受热、光照、接触空气 6.聚合危害 聚合 |
| 水溶解性 | MISCIBLE |
| 分子结构 | 1、摩尔折射率:17.23 2、摩尔体积(cm3/mol):67.7 3、等张比容(90.2K):162.3 4、表面张力(dyne/cm):32.8 5、极化率(10-24cm3):6.83 |
| 计算化学 | 1、疏水参数计算参考值(XlogP):0.3 2、氢键供体数量:1 3、氢键受体数量:2 4、可旋转化学键数量:1 5、拓扑分子极性表面积(TPSA):37.3 6、重原子数量:5 7、表面电荷:0 8、复杂度:55.9 9、同位素原子数量:0 10、确定原子立构中心数量:0 11、不确定原子立构中心数量:0 12、确定化学键立构中心数量:0 13、不确定化学键立构中心数量:0 14、共价键单元数量:1 |
| 更多 | 1.性状:无色液体,有刺激性气味。 2.熔点(℃):13 3.沸点(℃):141 4.相对密度(水=1):1.05 5.相对蒸气密度(空气=1):2.45 6.饱和蒸气压(kPa):1.33(39.9℃) 7.燃烧热(kJ/mol):-1366.9 8.临界压力(MPa):5.66 9.辛醇/水分配系数:0.36 10.闪点(℃):54(CC);54.5(OC) 11.引燃温度(℃):360 12.爆炸上限(%):8.0 13.爆炸下限(%):2.4 14.溶解性:与水混溶,可混溶于乙醇、乙醚。 15.黏度(mPa·s,25ºC):1.149 16.汽化热(KJ/mol):45.6 17.熔化热(KJ/mol,13ºC):11.1 18.相对密度(20℃,4℃):1.050 19.相对密度(25℃,4℃):1.044 20.常温折射率(n20):1.422 21.溶度参数(J·cm-3)0.5:26.229 22.van der Waals面积(cm2·mol-1):6.000×109 23.van der Waals体积(cm3·mol-1):39.930 24.液相标准燃烧热(焓)(kJ·mol-1):-1368.43 25.液相标准声称热(焓)( kJ·mol-1):-383.76 26.液相标准熵(J·mol-1·K-1) :226.4 27.液相标准热熔(J·mol-1·K-1):144.2 28.气相标准燃烧热(焓)(kJ·mol-1):-1428.7 29.气相标准声称热(焓)( kJ·mol-1) :-323.5 30.气相标准熵(J·mol-1·K-1) :307.73 31.气相标准生成自由能( kJ·mol-1):-271.0 32.气相标准热熔(J·mol-1·K-1):81.80 |
2.对环境的影响: 一、健康危害 侵入途径:吸入、食入、经皮吸收。 健康危害:本品对皮肤、眼睛和呼吸道有强烈刺激作用。 二、毒理学资料及环境行为 毒性:属低毒类。
急性毒性:LD 502520mg/kg(大鼠经口);950mg/kg(兔经皮);LC 505300mg/m 3,2小时(小鼠吸入) 致突变性:细胞遗传学分析:小鼠淋巴细胞450mg/L。 生殖毒性:大鼠腹腔最低中毒剂量(TDL 0):73216ug/kg(孕5~15天),致胚胎毒性,肌肉骨骼发育异常。 致癌性:IARC致癌性评论:动物、人类皆无可靠数据。 危险特性:其蒸气与空气形成爆炸性混合物,遇明火、高热能引起燃烧爆炸。与氧化剂能发生强烈反应。若遇高热,可能发生聚合反应,出现大量放热现象,引起容器破裂和爆炸事故。 燃烧(分解)产物:一氧化碳、二氧化碳。 3.现场应急监测方法:4.实验室监测方法:气相色谱法《气相色谱法测定环境大气中丙烯酸酯类化合物》顾海东等,上海环境监测,1998(1)P115~16 5.环境标准:
6.应急处理处置方法:一、泄漏应急处理 疏散泄漏污染区人员至安全区,禁止无关人员进入污染区,切断火源。建议应急处理人员戴自给式呼吸器,穿化学防护服。不要直接接触泄漏物,在确保安全情况下堵漏。喷水雾能减少蒸发但不要使水进入储存容器内。用沙土或其它不燃性吸附剂混合吸收,然后收集运至废物处理场所处置。如大量泄漏,利用围堤收容,然后收集、转移、回收或无害处理后废弃。 二、防护措施 呼吸系统防护:空气中浓度超标时,应该佩带防毒面具。紧急事态抢救或逃生时,佩带自给式呼吸器。 眼睛防护:戴化学安全防护眼镜。 防护服:穿工作服(防腐材料制作)。 手防护:戴橡皮手套。 其它:工作后,淋浴更衣。注意个人清洁卫生。 三、急救措施 皮肤接触:脱去污染的衣着,立即用水冲洗至少15分钟。 眼睛接触:立即提起眼睑,用流动清水或生理盐水冲洗至少15分钟。 吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。必要时进行人工呼吸。就医。 食入:误服者给饮大量温水,催吐,就医。 灭火方法:雾状水、二氧化碳、砂土、抗溶性泡沫。 |
|
丙烯酸毒理学数据: 1.急性毒性 LD50:2520mg/kg(大鼠经口);2400mg/kg(小鼠经口);950mg/kg(兔经皮) LC50:1200ppm(大鼠吸入,4h);5300mg/m3(小鼠吸入,2h) 2.刺激性 家兔经皮:500mg,重度刺激(开放性刺激试验)。 家兔经眼:250μg(24h),重度刺激。 3.致突变性 细胞遗传学分析:小鼠淋巴细胞450mg/L。哺乳动物体细胞突变:小鼠淋巴细胞500mg/L。细胞遗传学分析:仓鼠卵巢116mg/L。 4.致癌性 IARC致癌性评论:G3,对人及动物致癌性证据不足。 5.其他 大鼠腹腔最低中毒剂量(TDLo):73216μg/kg(孕5~15d),致胚胎毒性,肌肉骨骼发育异常。 丙烯酸生态学数据: 1.生态毒性 IC50:41mg/L(72h)(藻类) 2.生物降解性 好氧生物降解(h):24~168 厌氧生物降解(h):672~4320 3.非生物降解性 光解最大光吸收(nm):250 空气中光氧化半衰期(h):2.5~23.8 |
| 符号 |




GHS02, GHS05, GHS07, GHS09 |
|---|---|
| 信号词 | Danger |
| 危害声明 | H226-H302 + H312 + H332-H314-H335-H400 |
| 警示性声明 | P210-P261-P273-P303 + P361 + P353-P304 + P340 + P310-P305 + P351 + P338 |
| 个人防护装备 | Faceshields;full-face respirator (US);Gloves;Goggles;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| 危害码 (欧洲) | C:Corrosive |
| 风险声明 (欧洲) | R10;R20/21/22;R35;R50 |
| 安全声明 (欧洲) | S26-S36/37/39-S45-S61 |
| 危险品运输编码 | UN 2218 8/PG 2 |
| WGK德国 | 1 |
| RTECS号 | AS4375000 |
| 包装等级 | II |
| 危险类别 | 8 |
| 海关编码 | 2916110000 |
| 丙烯酸上游产品 10 | |
|---|---|
| 丙烯酸下游产品 10 | |
1.氰乙醇法 该法以氯乙醇和氰化钠为原料,反应生成氰乙醇,氰乙醇在硫酸存在下于175℃水解生成丙烯酸:若水解反应在甲醇中进行,则生成丙烯酸甲酯。
2.丙烯腈水解法 丙烯腈先以硫酸水解生成丙烯酰胺的硫酸盐,再水解生成丙烯酸,副产硫酸氢铵。此法在美国罗姆-哈斯公司得到了很大发展。
第一步水解温度为90~100℃。向丙烯腈中加入稍稍过量的55%~85%的硫酸,1h后丙烯腈即完全转化;然后加水进行第二次水解,并将反应温度提高到125~135℃;水解产物经减压蒸馏而得丙烯酸。此法实际上是早期氰乙醇法的发展。由于水解后生成的副产品酸性硫酸铵处理困难,原料丙烯腈的价格较贵,因而影响生产成本。
3.高压雷佩法将溶于四氢呋喃中乙炔,在溴化镍和溴化铜组成的催化剂存在下,与一氧化碳和水反应,制得丙烯酸。此法的特点是:用四氢呋喃为溶剂,可以减少高压处理乙炔的危险;同时催化剂不用原雷佩法所用的羰基镍,只需用镍盐。将丙烯与空气及水蒸气按一定摩尔比混合,在钼-铋等复合催化剂存在下,反应温度310-470℃,常压氧化制得丙烯醛,收率达90%。再将丙烯醛与空气及水蒸气按一定摩尔比混合,在钼-钒等复合催化剂存在下,反应温度300-470℃,常压氧化制得丙烯酸,收率可达98%。此法分一步和两步法。一步法是丙烯在一个反应器内氧化生成丙烯酸;两步法是丙烯先在第一反应器内氧化生成丙烯醛,丙烯醛再进入第二反应器氧化生成丙烯酸。两步法根据反应器结构,又分固定床和流化床法两种。丙烯酸的工业生产方法中,氰乙醇法,高压雷佩法已经基本淘汰,以前采用的以乙酸为原料裂解为乙烯酮,然后与无水甲醛反应生成丙内酯,再与热磷酸接触异构为丙烯酸。称烯酮法或β-丙内酯法也基本淘汰,丙烯腈法只有少数老装置采用。目前工业上采用的主要是改良雷佩法和丙烯氧化法,而后者更为普通且最有发展前途。专利报道中,还有丙酸为原料的生产方法。
4.β -丙内酯法 此法原料为乙烯酮,故又称乙烯酮法,其反应式如下:
先将乙酸裂解为乙烯酮,然后与无水甲醛反应生成β 丙内酯;用作催化剂在140~180℃、2.5~25MPa下,丙内酯再与热的100%磷酸接触,异构为丙烯酸。用β -丙内酯法生产丙烯酸,产品纯度高,收率亦较高,副产物和未反应物料能循环使用,并适于连续生产,但它需用乙酸为原料,特别是由于丙内酯被认为是一种致癌物质,故此法已不在工业上采用。
5.丙烯氧化法 其反应式如下:
将丙烯与空气及水蒸气按一定摩尔比混合,在钼铋系复合催化剂存在下,氧化制得丙烯醛,再将丙烯醛与空气及水蒸气按一定摩尔比混合,在钼-钒-钨系复合催化剂存在下,氧化制得丙烯酸。此法根据反应器结构,又分固定床法和流化床法两种。除美国索亥俄法采用流化床外,其他都采用列管式固定床。
① 固定床法。制法是:第一反应器进料丙烯含量为4%~7%,水蒸气20%~50%,其余为空气,空速1300~2600h-1,反应温度320~340℃,压力0.1~0.3MPa;第二反应器空速为1800~3600h-1,反应温度280~300℃,压力0.1~0.2MPa,丙烯和丙烯醛的转化率都在95%以上,丙烯酸的选择性以丙烯计为85%~90%。工艺过程为:使丙烯、水蒸气与经过预热的空气混合后进入第一反应器。丙烯被氧化成丙烯醛。再进入第二反应器反应,得到丙烯酸。第一、第二反应器均为列管式反应器,用熔盐作热载体,从第二反应器出来的反应气与原料空气换热后进入急冷塔,与塔顶加入的水逆向接触,获得含量为20%~30%的丙烯酸水溶液。该水溶液进入萃取塔,以乙酸丁酯或二甲苯为萃取剂,使水与丙烯酸分离。富含水的萃取液从萃取塔塔顶出来,进入溶剂回收塔,将萃取剂从塔顶蒸出,送回萃取塔循环使用。塔底排出废水。萃取塔中的萃余液进入溶剂蒸馏塔。从塔顶蒸出溶剂 (萃取剂) ,送回萃取塔循环使用;塔底得到粗丙烯酸,再经脱去轻组分和重组分后得到丙烯酸产品。丙烯经气相接触氧化反应制造丙烯酸过程中,除产物丙烯酸外,还存有微量丙烯醛、乙酸、戊酮酸、蚁酸以及其他醛类杂质。醛类是丙烯氧化副产物或由于丙烯原料中含有的杂质氧化而生成的,如乙醛、甲醛、苯甲醛、糠醛、丙烯醛等,含有这些副产物的反应气体,经冷却、抽提蒸馏后,残留于丙烯酸产品中。采用常规方法精制的丙烯酸产品中,仍含有约 (50~500) ×10-6的醛物质。为了适应高纯丙烯酸需要,北京东方化工厂以该厂聚合级丙烯酸为原料,开发出一种制备高纯丙烯酸的方法,使其总醛含量小于5×10-6,达到或超过国外有关文献报道的数据要求 ( 国外小于10×10-6) 。其实验方法是:向烧瓶中加入一定量的聚合级丙烯酸,添加试剂DL,在常压下经10~80℃范围处理后,再进入填料塔中处理蒸馏,塔釜中温度为60~80℃,塔顶温度为50~70℃,真空93.33~99.99KPa,采用补加阻聚剂及气相阻聚剂方法,可防止丙烯酸在精馏过程中聚合。用此法收集的蒸馏品即为高纯丙烯酸。
② 流化床法。制法是丙烯、空气、水经过第一沸腾床反应器生成丙烯醛,再进入第二沸腾床反应器生成丙烯酸,然后经喷淋、冷却、萃取蒸馏,再在减压塔中脱除乙酸而得丙烯酸。氧化混合物配比为丙烯∶空气∶水=1∶12∶8( 摩尔比) 。第一沸腾床反应器温度370℃,接触时间2s;第二沸腾床反应器温度260℃,接触 时 间2.25s 。丙 烯 转 化 率75%~80%,总 收 率40%,丙烯酸含量97%,平均含量93%。国内在第一反应器中采用七元组分 ( 钼-钒-磷-铁- 钴-镍-钾)的催化剂,丙烯氧化制丙烯醛;在第二反应器中采用三元组分 ( 钼- 钒-钨)的催化剂,丙烯醛氧化制丙烯酸,当丙烯∶空气∶水=1∶10∶6,接触时间5.5s,线速度0.6m/s反应温度:一段为370-390 ℃,二段为270~300℃时,以进料丙烯计,一段丙烯转化率为78.7%~87%,丙烯 醛 收 率 为 51.9%~57.2%,二 段 丙 烯 转 化 率 为79.3%~89.4%,丙烯酸收率为48.3%~49.8%,丙烯酸的空时收率为55~60kg/(m3催化剂·h) 。
| 海关编码 | 2916110000 |
|---|---|
| 中文概述 | 2916110000 丙烯酸及其盐. 增值税率:17.0% 退税率:9.0% 监管条件:无 最惠国关税:6.5% 普通关税:30.0% |
| 申报要素 | 品名, 成分含量, 用途, 丙烯酸、丙烯酸盐或酯应报明包装 |
| Summary | 2916110000 salts of acrylic acid VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Curcumin delivery from poly(acrylic acid-co-methyl methacrylate) hollow microparticles prevents dopamine-induced toxicity in rat brain synaptosomes.
Int. J. Pharm. 486 , 259-67, (2015) The potential of poly(methyl methacrylate-co-acrylic acid) (PMMA-AA) copolymers to form hollow particles and their further formulation as curcumin delivery system have been explored. The particles wer... |
|
|
Air to lung partition coefficients for volatile organic compounds and blood to lung partition coefficients for volatile organic compounds and drugs.
Eur. J. Med. Chem. 43 , 478-85, (2008) Values of in vitro gas to lung partition coefficients, K(lung), of VOCs have been collected from the literature. For 44 VOCs, application of the Abraham solvation equation to log K(lung) yielded a cor... |
|
|
Poly(acrylic acid)-grafted fluoropolymer films for highly sensitive fluorescent bioassays.
ACS Appl. Mater. Interfaces 5(6) , 2155-60, (2013) In this study, a facile and effective method for the surface functionalization of inert fluoropolymer substrates using surface grafting was demonstrated for the preparation of a new platform for fluor... |
| monoethylene carboxylic acid |
| Acrylic acid |
| propenoic acid |
| EINECS 201-177-9 |
| 2-propenoic acid |
| vinylcarboxylic acid |
| MFCD00004367 |

 CAS号107-02-8
CAS号107-02-8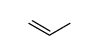 CAS号187737-37-7
CAS号187737-37-7 CAS号74-98-6
CAS号74-98-6 CAS号56-81-5
CAS号56-81-5 CAS号107-93-7
CAS号107-93-7 CAS号54509-73-8
CAS号54509-73-8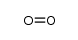 CAS号80937-33-3
CAS号80937-33-3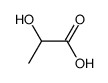 CAS号849585-22-4
CAS号849585-22-4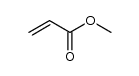 CAS号292638-85-8
CAS号292638-85-8 CAS号503-66-2
CAS号503-66-2 CAS号52684-34-1
CAS号52684-34-1 CAS号111709-01-4
CAS号111709-01-4 CAS号106664-87-3
CAS号106664-87-3 CAS号141-76-4
CAS号141-76-4 CAS号302-84-1
CAS号302-84-1 CAS号75-04-7
CAS号75-04-7 CAS号302-72-7
CAS号302-72-7 CAS号74-89-5
CAS号74-89-5 CAS号56-40-6
CAS号56-40-6 CAS号107-95-9
CAS号107-95-9


