Cortisone-d2
更新时间:2025-08-26 07:12:47
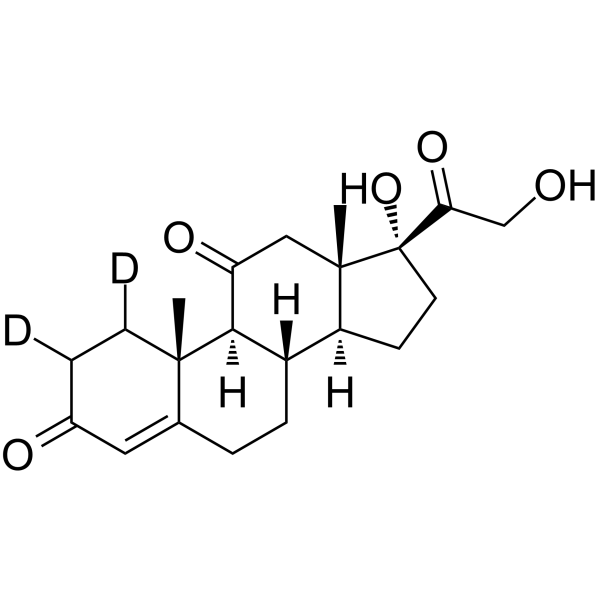
Cortisone-d2结构式

|
常用名 | Cortisone-d2 | 英文名 | Cortisone-d2 |
|---|---|---|---|---|
| CAS号 | 2687960-86-5 | 分子量 | 362.46 | |
| 密度 | N/A | 沸点 | N/A | |
| 分子式 | C21H26D2O5 | 熔点 | N/A | |
| MSDS | N/A | 闪点 | N/A |
Cortisone-d2用途可的松-d2是氘标记的可的松。皮质醇(17-羟基-11-脱氢皮质酮),皮质醇(一种糖皮质激素)的氧化代谢产物。可的松是一种免疫抑制剂和抗炎剂。高浓度可的松可部分干预糖皮质激素与糖皮质激素受体的结合[1][3][4][5]。 |
| 英文名 | Cortisone-d2 |
|---|
| 描述 | 可的松-d2是氘标记的可的松。皮质醇(17-羟基-11-脱氢皮质酮),皮质醇(一种糖皮质激素)的氧化代谢产物。可的松是一种免疫抑制剂和抗炎剂。高浓度可的松可部分干预糖皮质激素与糖皮质激素受体的结合[1][3][4][5]。 |
|---|---|
| 相关类别 | |
| 体外研究 | 氢、碳和其他元素的稳定重同位素已被纳入药物分子中,主要作为药物开发过程中定量的示踪剂。氘化因其可能影响药物的药代动力学和代谢特征而受到关注[1]。 |
| 参考文献 |
| 分子式 | C21H26D2O5 |
|---|---|
| 分子量 | 362.46 |
| InChIKey | MFYSYFVPBJMHGN-UFPKMYEYSA-N |
| SMILES | CC12CCC(=O)C=C1CCC1C2C(=O)CC2(C)C1CCC2(O)C(=O)CO |

