乙酸阿比特龙酯
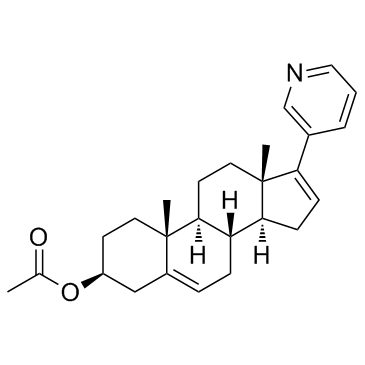
乙酸阿比特龙酯结构式

|
常用名 | 乙酸阿比特龙酯 | 英文名 | Abiraterone Acetate |
|---|---|---|---|---|
| CAS号 | 154229-18-2 | 分子量 | 391.546 | |
| 密度 | 1.1±0.1 g/cm3 | 沸点 | 506.7±50.0 °C at 760 mmHg | |
| 分子式 | C26H33NO2 | 熔点 | 127-130°C | |
| MSDS | 中文版 美版 | 闪点 | 260.2±30.1 °C | |
| 符号 |

GHS08 |
信号词 | Danger |
乙酸阿比特龙酯用途Abiraterone acetate是口服,有效,选择性和不可逆的 CYP17 抑制剂。 |
| 中文名 | 乙酸阿比特龙酯 |
|---|---|
| 英文名 | abiraterone acetate |
| 中文别名 | 醋酸阿比特龙 | (3BETA)-17-(3-吡啶基)-雄甾-5,16-二烯-3-乙酸酯 | 阿比特龙乙酸盐 | 醋酸阿比特龙酯 |
| 英文别名 | 更多 |
| 描述 | Abiraterone acetate是口服,有效,选择性和不可逆的 CYP17 抑制剂。 |
|---|---|
| 相关类别 | |
| 靶点 |
CYP17[1] |
| 体外研究 | 阿比特龙(Abi)乙酸酯是抗癌剂阿比特龙的酯前药,对于17,20-裂解酶和17α-羟化酶显示IC 50值为15 nM和2.5 nM(CYP17是具有17α-羟化酶和17的双功能酶, 20-裂解酶活性)。阿比特龙抑制人17,20-裂解酶和17α-羟化酶,IC50分别为27和30 nM [1]。用阿齐拉酮≥5μM剂量显着抑制AR阳性前列腺癌细胞系LNCaP和VCaP的增殖[2]。阿比特龙抑制重组人3βHSD1和3βHSD2活性,竞争性Ki值为2.1和8.8μM。 10μM阿比特龙足以完全阻断两种细胞系中5α-二酮和DHT的合成。阿比特龙的治疗显着抑制稳健生长子集中的CRPC进展,有效地使治疗4周内肿瘤生长达到上限(P <0.00001) [3]。 |
| 体内研究 | 阿比特龙(Abi)乙酸盐可延长去势抵抗性前列腺癌(CRPC)的存活率。 LNCaP中阿比特龙抑制[3H] -脱氢表雄酮(DHEA)消耗和Δ4-雄烯二酮(AD)积累,IC50 <1μM。先前显示0.5mmol/kg/d阿比特龙治疗剂量产生约0.5至1μM的血清浓度。对照组的异种移植肿瘤生长变化很大,一些肿瘤生长缓慢,只有一部分肿瘤表现出强劲的生长[3]。 |
| 细胞实验 | 将LNCaP和VCaP细胞接种在96孔板中,并在补充CSS的无酚红或FBS补充的培养基中生长7天。在接种后24和96小时,用阿比特龙(5μM和10μM)处理细胞,并通过添加CellTiter Glo并测量发光在第7天测定细胞活力[2]。 |
| 动物实验 | 小鼠[3]通过手术切除睾丸切除6至8周龄的雄性NOD / SCID小鼠,并植入5mg 90天持续释放DHEA小丸以模拟具有人肾上腺生理学的CRPC。两天后,用Matrigel皮下注射7×106个LAPC4细胞。肿瘤尺寸每周测量2至3次,体积计算为长×宽×高×0.52。一旦肿瘤达到300mm 3,将小鼠随机分配到载体或阿比特龙治疗组。阿比特龙组小鼠用5 mL / kg腹腔注射0.5 mmol / kg / d(0.1 mL 5%苄醇和95%红花油溶液)和对照小鼠仅用载体治疗,每日一次,每周5天以上持续4周(每次治疗n = 8只小鼠)。基于混合效应模型,通过ANOVA评估阿比特龙和媒介物治疗组之间的统计学显着性。 |
| 参考文献 |
| 密度 | 1.1±0.1 g/cm3 |
|---|---|
| 沸点 | 506.7±50.0 °C at 760 mmHg |
| 熔点 | 127-130°C |
| 分子式 | C26H33NO2 |
| 分子量 | 391.546 |
| 闪点 | 260.2±30.1 °C |
| 精确质量 | 391.251129 |
| PSA | 39.19000 |
| LogP | 6.55 |
| InChIKey | UVIQSJCZCSLXRZ-UBUQANBQSA-N |
| SMILES | CC(=O)OC1CCC2(C)C(=CCC3C2CCC2(C)C(c4cccnc4)=CCC32)C1 |
| 外观性状 | 固体;White to Light yellow to Light orange powder to crystal |
| 蒸汽压 | 0.0±1.3 mmHg at 25°C |
| 折射率 | 1.584 |
| 储存条件 | -20°C Freezer |
| 符号 |

GHS08 |
|---|---|
| 信号词 | Danger |
| 危害声明 | H360-H372 |
| 警示性声明 | P201-P260-P280-P308 + P313 |
| 靶器官 | Endocrine system |
| 危害码 (欧洲) | Xi |
| 危险品运输编码 | NONH for all modes of transport |
| RTECS号 | BV7992100 |
| 海关编码 | 2933399090 |
| 海关编码 | 2933399090 |
|---|---|
| 中文概述 | 2933399090. 其他结构含非稠合吡啶环的化合物. 增值税率:17.0%. 退税率:13.0%. 监管条件:无. 最惠国关税:6.5%. 普通关税:20.0% |
| 申报要素 | 品名, 成分含量, 用途, 乌洛托品请注明外观, 6-己内酰胺请注明外观, 签约日期 |
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy.
J. Clin. Oncol. 28(9) , 1481-8, (2010) Abiraterone acetate is a prodrug of abiraterone, a selective inhibitor of CYP17, the enzyme catalyst for two essential steps in androgen biosynthesis. In castration-resistant prostate cancers (CRPCs),... |
|
|
Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate.
J. Clin. Oncol. 28(9) , 1489-95, (2010) The principal objective of this trial was to evaluate the antitumor activity of abiraterone acetate, an oral, specific, irreversible inhibitor of CYP17 in docetaxel-treated patients with castration-re... |
|
|
Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer.
J. Clin. Oncol. 28(9) , 1496-501, (2010) Persistence of ligand-mediated androgen receptor signaling has been documented in castration-resistant prostate cancers (CRPCs). Abiraterone acetate (AA) is a potent and selective inhibitor of CYP17, ... |
| 17-(3-pyridyl)-5,16-androstadien-3beta-acetate |
| Abiraterone acetate |
| Androsta-5,16-dien-3-ol, 17-(3-pyridinyl)-, acetate (ester), (3β)- |
| Zytiga |
| (3β)-17-(3-Pyridinyl)androsta-5,16-dien-3-yl acetate |
| (3β)-17-(pyridin-3-yl)androsta-5,16-dien-3-yl acetate |
| Abiraterone (acetate) |

 CAS号115375-60-5
CAS号115375-60-5 CAS号1692-25-7
CAS号1692-25-7 CAS号108-24-7
CAS号108-24-7 CAS号154229-19-3
CAS号154229-19-3 CAS号75-36-5
CAS号75-36-5 CAS号626-55-1
CAS号626-55-1![[10,13-dimethyl-17-[(4-methylphenyl)sulfonylhydrazinylidene]-1,2,3,4,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-3-yl] acetate结构式](https://image.chemsrc.com/caspic/478/89359-48-8.png) CAS号89359-48-8
CAS号89359-48-8 CAS号853-23-6
CAS号853-23-6 CAS号89878-14-8
CAS号89878-14-8 CAS号53-43-0
CAS号53-43-0
