普瑞巴林

普瑞巴林结构式

|
常用名 | 普瑞巴林 | 英文名 | Pregabalin |
|---|---|---|---|---|
| CAS号 | 148553-50-8 | 分子量 | 159.226 | |
| 密度 | 1.0±0.1 g/cm3 | 沸点 | 274.0±23.0 °C at 760 mmHg | |
| 分子式 | C8H17NO2 | 熔点 | 194-196ºC | |
| MSDS | 中文版 美版 | 闪点 | 119.5±22.6 °C | |
| 符号 |


GHS05, GHS08 |
信号词 | Danger |
普瑞巴林用途该药是在开发的癫痫治疗药中最有希望的一个药物,疗效更好和给药更方便。也可以用于治疗疼痛和焦虑。 |
| 中文名 | 普瑞巴林 |
|---|---|
| 英文名 | Pregabalin |
| 中文别名 | (S)-3-氨甲基-5-甲基己酸 | 普瑞巴林 | (3S)-3-氨甲基-5-甲基己酸 |
| 英文别名 | 更多 |
| 密度 | 1.0±0.1 g/cm3 |
|---|---|
| 沸点 | 274.0±23.0 °C at 760 mmHg |
| 熔点 | 194-196ºC |
| 分子式 | C8H17NO2 |
| 分子量 | 159.226 |
| 闪点 | 119.5±22.6 °C |
| 精确质量 | 159.125931 |
| PSA | 63.32000 |
| LogP | 1.12 |
| 外观性状 | 白色或近乎于白色结晶粉末 |
| 蒸汽压 | 0.0±1.2 mmHg at 25°C |
| 折射率 | 1.465 |
| 储存条件 | Store at RT |
| 符号 |


GHS05, GHS08 |
|---|---|
| 信号词 | Danger |
| 危害声明 | H318-H361 |
| 警示性声明 | P280-P305 + P351 + P338 |
| 危害码 (欧洲) | Xn,T,F |
| 风险声明 (欧洲) | 63-48/22-39/23/24/25-23/24/25-11 |
| 安全声明 (欧洲) | 22-36/37-45-16-7 |
| 危险品运输编码 | NONH for all modes of transport |
| 海关编码 | 2915900090 |
| 海关编码 | 2922499990 |
|---|---|
| 中文概述 | 2922499990 其他氨基酸及其酯及它们的盐(含有一种以上含氧基的除外). 增值税率:17.0% 退税率:9.0% 监管条件:AB(入境货物通关单,出境货物通关单) 最惠国关税:6.5% 普通关税:30.0% |
| 申报要素 | 品名, 成分含量, 用途, 乙醇胺及其盐应报明色度, 乙醇胺及其盐应报明包装 |
| 监管条件 | A.入境货物通关单 B.出境货物通关单 |
| 检验检疫 | P.进境动植物、动植物产品检疫 Q.出境动植物、动植物产品检疫 R.进口食品卫生监督检验 S.出口食品卫生监督检验 M.进口商品检验 N.出口商品检验 |
| Summary | HS:2922499990 other amino-acids, other than those containing more than one kind of oxygen function, and their esters; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) MFN tariff:6.5% General tariff:30.0% |
|
Development of a SPE-HPLC-MS/MS method for the determination of most prescribed pharmaceuticals and related metabolites in urban sewage samples.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 990 , 23-30, (2015) Based on regional prescription data several pharmaceuticals with variable amounts of prescription and corresponding metabolites were selected and analyzed in influent and effluent samples of the sewag... |
|
|
Effect of the gastrointestinal prokinetic agent erythromycin on the pharmacokinetics of pregabalin controlled-release in healthy individuals: a phase I, randomized crossover trial.
Clin. Drug Investig. 35(5) , 299-305, (2015) The controlled-release (CR) formulation of pregabalin is designed to remain in the stomach for a prolonged period while slowly releasing pregabalin for absorption in the small intestine. This study ev... |
|
|
Evaluation of the matrix effect of different sample matrices for 33 pharmaceuticals by post-column infusion.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 1000 , 84-94, (2015) Matrix effects that occur during quantitative measurement by liquid chromatography mass spectrometry specifically when using electrospray ionization are a widely recognized phenomenon. Sample matrix c... |
| (S)-3-Aminomethyl-5-methyl-hexanoic acid |
| EINECS 200-659-6 |
| (S)-3-(Aminomethyl)-5-methylhexanoic acid CI-1008 PD-144723 |
| PREDNISOLONESODIUMPHOSPHATE |
| Hexanoic acid, 3-(aminomethyl)-5-methyl-, (3S)- |
| Pregablin |
| (S)-3-(Aminomethyl)-5-methylhexanoic acid |
| Pregabalin |
| Lyrica |
| prégabaline |
| (S)-3-Isobutyl GABA |
| MFCD00917044 |
| (S)-(+)-3-aminomethyl-5-methylhexanoic acid |
| (3S)-3-(Aminomethyl)-5-methylhexanoic acid |

 CAS号181289-39-4
CAS号181289-39-4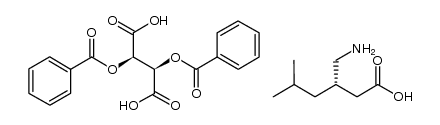 CAS号1078737-39-9
CAS号1078737-39-9 CAS号1310495-04-5
CAS号1310495-04-5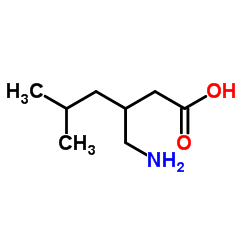 CAS号128013-69-4
CAS号128013-69-4 CAS号181289-33-8
CAS号181289-33-8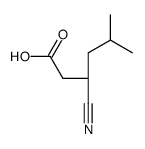 CAS号181289-37-2
CAS号181289-37-2
