丙烯腈
一般危化品
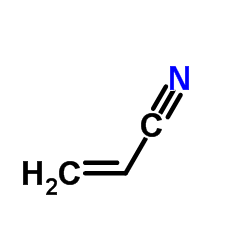
丙烯腈结构式
|
常用名 | 丙烯腈 | 英文名 | Acrylonitrile |
|---|---|---|---|---|
| CAS号 | 107-13-1 | 分子量 | 53.063 | |
| 密度 | 0.8±0.1 g/cm3 | 沸点 | 77.3±0.0 °C at 760 mmHg | |
| 分子式 | C3H3N | 熔点 | -83.5 °C | |
| MSDS | 中文版 美版 | 闪点 | 0.0±0.0 °C | |
| 符号 |



GHS02, GHS06, GHS08 |
信号词 | Danger |
丙烯腈用途【用途一】 用作色谱分析标准物质,也用于橡胶、塑料、有机合成及杀虫剂的制造 【用途二】
用于合成聚丙烯腈、尼龙66、丁腈橡胶、ABS树脂、聚丙烯酰胺、丙烯酸酯类等,也用作谷物烟熏剂 【用途三】 丙烯腈是杀菌剂溴菌腈、霜霉威,杀虫剂毒死蜱和杀虫双、杀螟丹的中间体,还可以制备二氯菊酸甲酯用以生产拟除虫菊酯,也是杀虫剂虫满腈的中间体。 【用途四】 丙烯腈是合成纤维,合成橡胶和合成树脂的重要单体。由丙烯腈制得聚丙烯腈纤维即(腈纶),其性能极似羊毛,因此也叫(合成羊毛)。丙烯腈与丁二烯共聚可制得丁腈橡胶,具有良好的耐油性,耐寒性,耐磨性,和电绝缘性能,并且在大多数化学溶剂,阳光和热作用下,性能比较稳定。丙烯腈与丁二烯,苯乙烯共聚制得ABS树脂,具有质轻,耐寒,抗冲击性能较好等优点。丙烯腈水解可制得丙烯酰胺和丙烯酸及其酯类。它们是重要的有机化工原料,丙烯腈还可电解加氢偶联制得己二腈,由己二腈加氢又可制得己二胺,己二胺是尼龙66原料。可制造抗水剂和胶粘剂等,也用于其他有机合成和医药工业中,并用作谷类熏蒸剂等。此外,本品也是一种非质子型极性溶剂。 更多
|
| 中文名 | 丙烯腈 |
|---|---|
| 英文名 | acrylonitrile |
| 中文别名 | 乙烯基氰 |
| 英文别名 | 更多 |
| 密度 | 0.8±0.1 g/cm3 |
|---|---|
| 沸点 | 77.3±0.0 °C at 760 mmHg |
| 熔点 | -83.5 °C |
| 分子式 | C3H3N |
| 分子量 | 53.063 |
| 闪点 | 0.0±0.0 °C |
| 精确质量 | 53.026550 |
| PSA | 23.79000 |
| LogP | 0.19 |
| 外观性状 | 透明液体 |
| 蒸汽密度 | 1.83 (vs air) |
| 蒸汽压 | 97.1±0.1 mmHg at 25°C |
| 折射率 | 1.385 |
| 储存条件 | 1.储存注意事项 通常商品加有稳定剂。储存于阴凉、通风良好的专用库房内,实行“双人收发、双人保管”制度。远离火种、热源。库温不宜超过37℃。包装要求密封,不可与空气接触。应与氧化剂、酸类、碱类、食用化学品分开存放,切忌混储。不宜大量储存或久存。采用防爆型照明、通风设施。禁止使用易产生火花的机械设备和工具。储区应备有泄漏应急处理设备和合适的收容材料。 2.本品采用铁桶包装。贮存容器要密封,仓库要有良好的通风,防止日晒,要远离硫酸、硝酸等强酸性物质。按“危险品规定”贮运。 |
| 稳定性 | 1.化学性质:化学性质活泼,能发生双键加成反应,与相应的含有活泼氢的无机或有机化合物反应制成一系列氰乙基化产物。在缺氧或暴露在可见光情况下易聚合,在浓碱存在下能强烈聚合。与还原剂发生激烈反应,放出有毒气体。蒸气与空气混合易形成爆炸性混合物,与氧化剂发生强烈反应,遇明火、高热会引起燃烧爆炸。见光、遇热、久贮易聚合,有燃烧爆炸危险。 2.本品极毒,对温血动物的毒性约为氰化氢的1/30。丙烯腈不仅蒸气有毒,而且附着于皮肤上也易经皮肤中毒。对小鼠静脉注射LD5015mg/kg,大鼠LD50为93mg/kg。长时间吸入稀丙烯腈蒸气,则能引起恶心、呕吐、头痛、疲倦和不适等症状。工作场所最高容许浓度为45mg/m3。生产设备要密闭,操作时要戴防护用具。丙烯腈若溅到衣服上应立即脱下衣服,溅及皮肤时用大量水冲洗。溅入眼内,需用流水冲洗15分钟以上。不慎吞入时,则用温盐水洗胃。如果中毒,应立即用硫代硫酸钠、亚硝酸钠进行静脉注射,并请医生诊治。 3.稳定性 稳定 4.禁配物 强氧化剂、碱类、酸类 5.避免接触的条件 受热、光照、接触空气 6.聚合危害 聚合 7.分解产物 氰化氢 |
| 水溶解性 | Soluble. 7.45 g/100 mL |
| 分子结构 | 1、摩尔折射率:15.58 2、摩尔体积(cm3/mol):66.5 3、等张比容(90.2K):148.8 4、表面张力(dyne/cm):25.0 5、介电常数: 6、偶极距(10-24cm3): 7、极化率:6.17 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:0 3.氢键受体数量:1 4.可旋转化学键数量:0 5.互变异构体数量:无 6.拓扑分子极性表面积23.8 7.重原子数量:4 8.表面电荷:0 9.复杂度:54.9 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1.性状:无色液体,有刺激性气味。 2.pH值:6~7.5(5%溶液) 3.熔点(℃):-83.6 4.沸点(℃):77.3 5.相对密度(水=1):0.81 6.相对蒸气密度(空气=1):1.83 7.饱和蒸气压(kPa):11.07(20℃) 8.燃烧热(kJ/mol):-1761.5 9.临界温度(℃):246 10.临界压力(MPa):3.54 11.辛醇/水分配系数:0.25 12.闪点(℃):-1(CC) 13.引燃温度(℃):481 14.爆炸上限(%):17.0 15.爆炸下限(%):3.0 16.溶解性:微溶于水,易溶于多数有机溶剂。 17.折射率(20ºC):1.3911 18.黏度(mPa·s,25ºC):0.34 |
2.对环境的影响: 一、健康危害 侵入途径:吸入、食入、经皮吸收。 健康危害:本品在体内析出氰根,抑制呼吸酶;对呼吸中枢有直接麻醉作用。急性中毒表现与氢氰酸相似。 急性中毒:以中枢神经系统症状为主,伴有上呼吸道和眼部刺激症状。轻度中毒有头晕、头木、意识蒙胧及口唇紫绀等。眼结膜及鼻、咽部充血。重者除上述症状加重外,出现四肢阵发性强直抽搐、昏迷。液体污染皮肤,可致皮炎,局部出现红斑、丘疹或水疱。 慢性中毒:尚无定论。长期接触,部分工人出现神衰综合征、低血压等。对肝脏影响未肯定。
二、毒理学资料及环境行为 毒性:属高毒类。 急性毒性:LD 5078mg/kg(大鼠经口);250mg/kg(兔经皮);人吸入300~500mg/m 3×5~10分钟,上呼吸道灼痛、流泪;人吸入35~200mg/m 3×20~45分钟,粘膜刺激。 亚急性和慢性毒性:大鼠吸入40mg/m 3×4小时/日×6日/周×40日,致死,肝坏死;大鼠经口0.1%饮水×13周,生长减慢,萎靡。 刺激性:家兔经眼:20mg(24小时),重度刺激。家兔经皮:500mg,轻度刺激。 致突变性:微生物致变突性:鼠伤寒沙门氏菌25µL/皿。哺乳动物体细胞突变性:人淋巴细胞25mg/L。 生殖毒性:大鼠经口最低中毒剂量(TDL 0):650mg/kg(孕6~15天),对雄性生育指数有影响,可引起胚胎毒性,肌肉骨骼发育异常。 致癌性:大鼠经口最小中毒剂量1700mg/kg(37周)胃癌。 污染来源:丙烯腈是重要的有机原料,主要用于橡胶合成(如丁腈橡胶)、塑料合成(如ABS,AS树脂、聚丙烯酰胺等)、有机合成、制造腈纶、尼龙66等膈成纤维、杀虫剂、抗水剂、粘合剂等;还用合适谷物烟薰剂。从事丙烯腈生产及以丙烯腈为原料合成和制造上述物质的企业均是丙烯腈污染的主要来源。腈纶纤维燃烧时可释放也丙烯腈。 丙烯腈在水中是不稳定的,水中浓度1mg/L以下时不影响生物降解。当水中丙烯腈的浓度为10mg/L时,经过一昼夜剩下46%,二昼夜只剩下19%,四昼夜剩下5%,六昼夜只剩下3.6%当水中丙烯脯的浓度为75mg/L时,经过二昼夜剩下30.5%,六昼夜时为起初数量的4.5%。空气中嗅觉阈值浓度为1.7~23ppm,水中嗅觉阈值浓度为0.0031~50.4mg/L。 危险特性:易燃,其蒸气与空气可形成爆炸性混合物。遇明火、高热易燃烧,并放出有毒气体。与氧化剂、强酸、强碱、胺类、溴反应剧烈。在火场高温下,能发生聚合放热,使容器破裂。 燃烧(分解)产物:一氧化碳、二氧化碳、氧化氮、氰化氢。 3.现场应急监测方法: 快速检测管法;便携式气相色谱法《突发性环境污染事故应急监测与处理处置技术》万本太主编 直接进水样气相色谱法;气体检测管法 气体速测管(德国德尔格公司产品) 4.实验室监测方法:
5.环境标准:
6.应急处理处置方法: 一、泄漏应急处理 迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿防毒服。尽可能切断泄漏源,防止进入下水道、排洪沟等限制性空间。小量泄漏:用活性炭或其它惰性材料吸收。也可用大量水冲洗,洗水稀释后放入废水系统。大量泄漏:构筑围堤或挖坑收容;用泡沫覆盖,降低蒸气灾害。喷雾状水冷却和稀释蒸气、保护现场人员、把泄漏物稀释成不燃物。用防爆泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。废弃物处置方法:焚烧法,焚烧炉要有后燃烧室,焚烧炉排出的氮氧化物通过洗涤器除去。化学法,用乙醇氢氧化钠处理,将其产物同大量水一起排入下水道。另外,从废水中回收丙烯腈也是一种可考虑的处理办法。 二、防护措施 呼吸系统防护:可能接触毒物时,必须佩戴过滤式防毒面具(全面罩)。紧急事态抢救或撤离时,佩戴空气呼吸器。 眼睛防护:呼吸系统防护中已作防护。 身体防护:穿连衣式胶布防毒衣。 手防护:戴橡胶手套。 其它:工作现场禁止吸烟、进食和饮水。工作毕,彻底清洗。单独存放被毒物污染的衣服,洗后备用。车间应配备急救设备及药品。作业人员应学会自救互救。 三、急救措施 皮肤接触:立即脱去被污染的衣着,用流动清水或5%硫代硫酸钠溶液彻底冲洗至少20分钟。就医。 眼睛接触:提起眼睑,用流动清水或生理盐水冲洗。就医。 吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。呼吸心跳停止时,立即进行人工呼吸(勿用口对口)和胸外心脏按压术。给吸入亚硝酸异戊酯,就医。 食入:饮足量温水,催吐,用1:5000高锰酸钾或5%硫代硫酸钠溶液洗胃。就医。 灭火方法:消防人员必须佩戴过滤式防毒面具(全面罩)或隔离式呼吸器、穿全身防火防毒服,在上风处灭火。灭火剂:二氧化碳、干粉、砂土。用水灭火无效,但须用水保持火场容器冷却。 |
|
丙烯腈毒理学数据: 1.急性毒性 LD50:78mg/kg(大鼠经口);27mg/kg(小鼠经口);148mg/kg(大鼠经皮);63mg/kg(兔经皮) LC50:333ppm(大鼠吸入,4h) 2.刺激性 家兔经皮:500mg,轻度刺激。 家兔经眼:20mg,重度刺激。 3.亚急性与慢性毒性 大鼠、豚鼠、兔和猫在330mg/m3下吸入,每天4h,每周5天,在4周内半数动物死亡;在220mg/m3浓度下,10周,除出现呼吸道症状外,未出现明显中毒症状。 4.致突变性 微生物致突变性:鼠伤寒沙门菌25μl/皿。哺乳动物体细胞突变性:人淋巴细胞25mg/L。 人吸入0.8mg/m3(146周),导致DNA损伤、精子形态学和细胞遗传学改变。 5.致畸性 雌性大鼠孕后8d腹腔内注射641mg/kg,导致仔鼠中枢神经系统和肌肉骨骼系统畸形。 6.致癌性 IARC致癌性评论:G2B,可疑人类致癌物。 7.其他 大鼠经口最低中毒剂量(TDLo):650mg/kg(孕6~15d),对雌性生育指数有影响,可引起胚胎毒性,肌肉骨骼发育异常。 丙烯腈生态学数据: 1.生态毒性 LC50:2.6mg/L(30d)(黑头呆鱼,静态);10.1mg/L(96h)(黑头呆鱼,动态);11.8mg/L(48h)(蓝鳃太阳鱼,静态);13mg/L(24h),7.6mg/L(48h)(水蚤) 2.生物降解性 好氧生物降解(h):30~552 厌氧生物降解(h):120~2208 3.非生物降解性 光解最大光吸收(nm):203 空气中光氧化半衰期(h):13.4~189 一级水解半衰期(h):1.06×107 4.生物富集性 蓝鳃太阳鱼,在流动水中接触48h,BCF为48。 |
| 符号 |



GHS02, GHS06, GHS08 |
|---|---|
| 信号词 | Danger |
| 危害声明 | H225-H301 + H311 + H331-H350-H370 |
| 警示性声明 | P201-P210-P260-P280-P301 + P310-P311 |
| 个人防护装备 | Faceshields;full-face respirator (US);Gloves;Goggles;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| 危害码 (欧洲) | F:Flammable |
| 风险声明 (欧洲) | R11;R23/24/25;R37/38;R41;R43;R45;R51/53 |
| 安全声明 (欧洲) | S53-S9-S16-S45-S61-S36/37 |
| 危险品运输编码 | UN 1093 3/PG 1 |
| WGK德国 | 3 |
| RTECS号 | AT5250000 |
| 包装等级 | I |
| 危险类别 | 3 |
| 海关编码 | 2926100000 |
| 丙烯腈上游产品 9 | |
|---|---|
| 丙烯腈下游产品 10 | |
1.氰乙醇法 环氧乙烷和氢氰酸在水和三甲胺的存在下反应得氰乙醇,然后以碳酸镁为催化剂,于200-280℃脱水制得丙烯腈,收率约75%。此法生产的丙烯腈纯度较高,但氢氰酸毒性大,成本也较高。2.乙炔法 乙炔和氢氰酸在氯化亚铜-氯化钾-氯化钠稀盐酸溶液的催化作用下在80-90℃反应得丙烯腈此法生产过程简单,收率良好,以氢氰酸计可达97%。但副反应多,产物精制较难,毒性也大,且原料乙炔价格高于丙烯,在技术和经济上落后于丙烯氨氧化法。1960年以前,该法是世界各国生产丙烯腈的主要方法。3.丙稀氨氧化法以丙烯、氨、空气和水为原料,按其一定量配比进入沸腾床或固定床反应器,在以硅胶作载体的磷钼鉍系或锑铁系催化剂作用下,在400-500℃温度和常压下,生成丙烯腈。然后经中和塔用稀硫酸除去未反应的氨,再经吸收塔用水吸收丙烯腈等气体,形成水溶液,使该水溶液经萃取塔分离乙腈,在脱氢氰酸塔除去氢氰酸,经脱水、精馏而得丙烯腈产品,其单程收率可达75%,副产品有乙腈、氢氰酸和硫铵。此法是目前最有工业生产价值的生产方法。
2.其制备方法主要用丙烯氨氧化法,以丙烯、氨气和空气中的氧气为原料,在催化剂存在下进行反应,催化剂主要是含磷、钼、铋系的化合物,丙烯、氨和空气的摩尔比为1∶(1~1.2)∶(1.8~2.3),反应温度为400~500℃,反应压力为常压,反应器为流化床。
该反应丙烯腈的单程收率60%~75%。
3.环氧乙烷法 由环氧乙烷和氢氰酸在水和三甲胺的存在下反应制得氰乙醇;然后以碳酸镁为催化剂,于200~280℃脱水制得丙烯腈:
5.丙烯氨氧化法 以丙烯、氨氧和空气中的氧为原料制得,主要副产物为氢氰酸、乙腈、丙烯醛、CO2和CO。主反应如下:
| 海关编码 | 2926100000 |
|---|---|
| 中文概述 | 2926100000 丙烯腈〔即2-丙烯腈、乙烯基氰〕。监管条件:X(有毒化学品环境管理放行通知单)。增值税率:17.0%。退税率:13.0%。最低关税:6.5%。普通关税:30.0% |
| 申报要素 | 品名, 成分含量, 用途 |
| 监管条件 | X.有毒化学品环境管理放行通知单 |
| Summary | 2926100000 acrylonitrile。supervision conditions:x(environment control release notice for poisonous chemicals)。VAT:17.0%。tax rebate rate:13.0%。MFN tarrif:6.5%。general tariff:30.0% |
|
Self-cleaning Metal Organic Framework (MOF) based ultra filtration membranes--a solution to bio-fouling in membrane separation processes.
Sci. Rep. 4 , 6555, (2014) Bio-fouling is a serious problem in many membrane-based separation processes for water and wastewater treatment. Current state of the art methods to overcome this are to modify the membranes with eith... |
|
|
Aptamer-functionalized solid phase microextraction-liquid chromatography/tandem mass spectrometry for selective enrichment and determination of thrombin.
Anal. Chim. Acta 845 , 45-52, (2014) In this publication, a novel solid phase microextraction (SPME) coating functionalized with a DNA aptamer for selective enrichment of a low abundance protein from diluted human plasma is described. Th... |
|
|
Double salts of ionic-liquid-based surfactants in microextraction: application of their mixed hemimicelles as novel sorbents in magnetic-assisted micro-dispersive solid-phase extraction for the determination of phenols.
Anal. Bioanal. Chem 407 , 8753-64, (2015) The use of mixed hemimicelles of ionic liquid (IL)-based surfactants in a magnetic-based micro-dispersive solid-phase extraction (m-μdSPE) approach is described. Not only is the symmetric monocationic... |
| MFCD00001927 |
| EINECS 203-466-5 |
| Acrylonitrile |
| 1-Methylethenylamine |
| Isopropenylamin |
| 1-Propen-2-amine |
| propenenitrile |
| acrylo-nitrile |
| 2-propenamine |
| cyanoethene |
| vinyl cyanide |
| prop-2-enenitrile |
| 2-Propenenitrile |
| propenamine-2 |

 CAS号107-02-8
CAS号107-02-8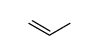 CAS号187737-37-7
CAS号187737-37-7 CAS号74-98-6
CAS号74-98-6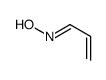 CAS号5314-33-0
CAS号5314-33-0 CAS号64-19-7
CAS号64-19-7 CAS号2417-90-5
CAS号2417-90-5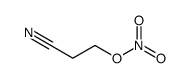 CAS号50434-02-1
CAS号50434-02-1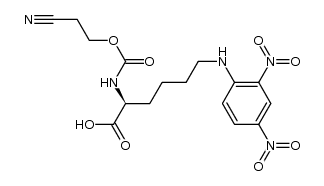 CAS号1202639-11-9
CAS号1202639-11-9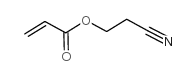 CAS号106-71-8
CAS号106-71-8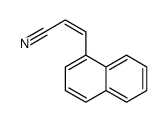 CAS号111686-30-7
CAS号111686-30-7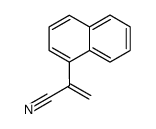 CAS号56477-58-8
CAS号56477-58-8![3-[2-[(2-chlorophenoxy)methyl]benzimidazol-1-yl]propanenitrile结构式](https://image.chemsrc.com/caspic/251/112055-56-8.png) CAS号112055-56-8
CAS号112055-56-8![3-[bis(2-methylpropyl)amino]propanenitrile结构式](https://image.chemsrc.com/caspic/154/112359-49-6.png) CAS号112359-49-6
CAS号112359-49-6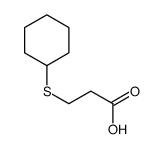 CAS号106664-87-3
CAS号106664-87-3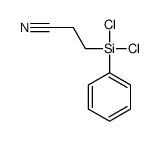 CAS号1077-57-2
CAS号1077-57-2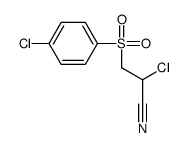 CAS号1015-44-7
CAS号1015-44-7 CAS号111140-91-1
CAS号111140-91-1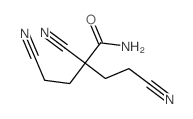 CAS号1112-50-1
CAS号1112-50-1 CAS号1112-27-2
CAS号1112-27-2


