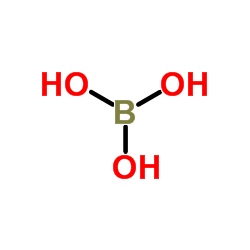
10043-11-5
| 中文名 | 氮化硼 |
|---|---|
| 英文名 | boron nitride |
| 英文别名 |
shobn
Nitriloborane Boron nitride boron niride EINECS 233-136-6 boron nitrides BN F15 elborr BN C MFCD00011317 BN B50 BN A01 uhp-ex wurzin bzn550 elbor |
| 密度 | 2.29 |
|---|---|
| 熔点 | 2700ºC (sublimes) |
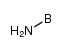
| 分子式 | BH2N |
| 分子量 | 24.818 |
| 精确质量 | 25.012381 |
| PSA | 23.79000 |
| 外观性状 | 白色粉末或半透明的晶体 |
| 折射率 | 1.728 |
| 储存条件 | 氮化硼贮存方法:应贮存在通风良好的干燥库房内,防止受潮。 氮化硼纤维贮存方法:贮存于通风良好、干燥库房内。空气中允许氮化硼最高浓度为6mg/m3。 |
| 稳定性 | 常温常压下稳定 避免光,明火,高温。与石墨性质相似,有良好的电绝缘性、导热性和耐化学腐蚀性。抗氧化性能甚强,在高温时也具有良好的润滑性,是一种优良的高温固体润滑剂。具有很强的中子吸收能力。化学性质稳定,对几乎所有的熔融金属都呈化学惰性。氮化硼不溶于冷水,与水煮沸时水解非常缓慢,产生少量硼酸和氨。与弱酸和强碱在室温时均不起反应,微溶于热酸,用熔融的氢氧化钾处理方能分解,氯气也只能在赤热的条件下方与它起反应。能耐高温至2000 ℃。具备便于机械加工的优良性能。立方晶型氮化硼硬度与金刚石相当,在1500~1600 ℃高温中稳定性优于金刚石。 氮化硼纤维:属多晶BN纤维。熔点300℃;直径4~6μm;密度1.8~1.9g/cm3;强度约2GPa;模量340~350GPa。氮化硼纤维与某些无机纤维相比,具有耐高温、耐化学腐蚀、电热性能好、耐辐射等优良特性。它的抗氧化温度比碳纤维、硼纤维的高,在惰性条件下,2500℃以上仍保持稳定;在氧化气氛中,850℃仍是稳定的。 六方晶系结晶。用高压高温处理所制得的是立方晶系结晶。六方晶系氮化硼的晶体结构和物理性质类似于石墨(但具有电绝缘性)。也称白石墨(white graphite)。莫氏硬度为2,具有优越的润滑性。在氧化气氛中约800℃,在惰性气体气氛中约3000℃是稳定的。不被熔融金属和矿渣所润湿。电绝缘性好,无毒,立方晶系氮化硼具有比金刚石还强的硬度。 |
| 分子结构 | 1、摩尔折射率:无可用的 2、摩尔体积(cm3/mol):无可用的 3、等张比容(90.2K):无可用的 4、表面张力(dyne/cm):无可用的 5、介电常数:无可用的 6、极化率(10-24cm3):无可用的 7、单一同位素质量:25.01238 Da 8、标称质量:25 Da 9、平均质量:24.8177 Da |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:0 3.氢键受体数量:1 4.可旋转化学键数量:0 5.互变异构体数量:无 6.拓扑分子极性表面积23.8 7.重原子数量:2 8.表面电荷:0 9.复杂度:10 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1. 性状:白色松散粉末。最常见为石墨晶格。有六方和立方两种晶型。有一种一氮化硼立方结晶的变体被认为是已知的最硬的物质。也有无定形变体。 2. 密度(g/mL,25/4℃): 2.26 3. 相对蒸汽密度(g/mL,空气=1):未确定 4. 熔点(ºC): 3000 5. 沸点(ºC,常压):未确定 6. 沸点(ºC,5.2kPa):未确定 7. 折射率:未确定 8. 闪点(ºC):未确定 9. 比旋光度(º):未确定 10. 自燃点或引燃温度(ºC):未确定 11. 蒸气压(kPa,25ºC):未确定 12. 饱和蒸气压(kPa,60ºC):未确定 13. 燃烧热(KJ/mol):未确定 14. 临界温度(ºC):未确定 15. 临界压力(KPa):未确定 16. 油水(辛醇/水)分配系数的对数值:未确定 17. 爆炸上限(%,V/V):未确定 18. 爆炸下限(%,V/V):未确定 19. 溶解性:具有抗化学侵蚀性质。不被无机酸和水侵蚀。在热浓碱中硼碳键被断开。微溶于热酸,不溶于冷水。 |
|
Version 1.4
Regulation (EC) No 1907/2006 1 - Product and Company Information Product NameBORON NITRIDE, POWDER, CA. 1 MICRON, 98% 2 - Hazards Identification SPECIAL INDICATION OF HAZARDS TO HUMANS AND THE ENVIRONMENT Irritating to eyes and respiratory system.
3 - Composition/Information on Ingredients Product NameCAS #EC noAnnex I Index Number BORON NITRIDE10043-11-5 233-136-6 None FormulaBN Molecular Weight 24,8200 AMU SynonymsBN 40SHP * Borazon * Boron mononitride * BZN 550 * Denka boron nitride GP * Denka GP * Elbor * Elbor LO 10B1-100 * Elboron * Elbor R * Elbor RM * Geksanit R * Hexanite R * Hexanit R * KBN-H10 * Kubonit * Kubonit KR * Sho BN * Sho BN HPS * SP 1 * SP 1 (Nitride) * Super mighty M * UHP-Ex * Wurzin 4 - First Aid Measures AFTER INHALATION If inhaled, remove to fresh air. If not breathing give artificial respiration. If breathing is difficult, give oxygen. AFTER SKIN CONTACT In case of contact, immediately wash skin with soap and copious amounts of water. AFTER EYE CONTACT In case of contact, immediately flush eyes with copious amounts of water for at least 15 minutes. AFTER INGESTION ALDRICHwww.molbase.com If swallowed, wash out mouth with water provided person is conscious. Call a physician. 5 - Fire Fighting Measures EXTINGUISHING MEDIA Suitable: Noncombustible. Use extinguishing media appropriate to surrounding fire conditions. SPECIAL RISKS Specific Hazard(s): Emits toxic fumes under fire conditions. SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS Wear self-contained breathing apparatus and protective clothing to prevent contact with skin and eyes. 6 - Accidental Release Measures PROCEDURE(S) OF PERSONAL PRECAUTION(S) Wear respirator, chemical safety goggles, rubber boots, and heavy rubber gloves. METHODS FOR CLEANING UP Sweep up, place in a bag and hold for waste disposal. Avoid raising dust. Ventilate area and wash spill site after material pickup is complete. 7 - Handling and Storage HANDLING Directions for Safe Handling: Do not breathe dust. Avoid contact with eyes, skin, and clothing. Avoid prolonged or repeated exposure. STORAGE Conditions of Storage: Keep container closed. Store in a cool dry place. SPECIAL REQUIREMENTS: Hygroscopic. 8 - Exposure Controls / Personal Protection ENGINEERING CONTROLS Safety shower and eye bath. Mechanical exhaust required. GENERAL HYGIENE MEASURES Wash thoroughly after handling. PERSONAL PROTECTIVE EQUIPMENT Special Protective Measures: Wear appropriate government approved respirator, chemical-resistant gloves, safety goggles, other protective clothing. 9 - Physical and Chemical Properties AppearanceColor: Faintly beige Form: Powder PropertyValueAt Temperature or Pressure ALDRICHwww.molbase.com pH N/A BP/BP RangeN/A MP/MP RangeN/A Flash PointN/A FlammabilityN/A Autoignition TempN/A Oxidizing Properties N/A Explosive Properties N/A Explosion LimitsN/A Vapor PressureN/A SG/Density2,2900 g/cm3 Partition Coefficient N/A ViscosityN/A Vapor DensityN/A Saturated Vapor Conc. N/A Evaporation RateN/A Bulk DensityN/A Decomposition Temp.N/A Solvent ContentN/A Water ContentN/A Surface TensionN/A ConductivityN/A Miscellaneous DataN/A SolubilityN/A 10 - Stability and Reactivity STABILITY Conditions of Instability: Moisture. Conditions to Avoid: Moisture. Materials to Avoid: Strong oxidizing agents. HAZARDOUS DECOMPOSITION PRODUCTS Hazardous Decomposition Products: Nature of decomposition products not known. HAZARDOUS POLYMERIZATION Hazardous Polymerization: Will not occur 11 - Toxicological Information RTECS NUMBER: ED7800000 SIGNS AND SYMPTOMS OF EXPOSURE To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly investigated. ROUTE OF EXPOSURE Skin Contact: May cause skin irritation. Eye Contact: Causes eye irritation. Inhalation: Material is irritating to mucous membranes and upper respiratory tract. Multiple Routes: May be harmful by inhalation, ingestion, or skin absorption. 12 - Ecological Information No data available. 13 - Disposal Considerations ALDRICHwww.molbase.com SUBSTANCE DISPOSAL Bury in a landfill site approved for the disposal of chemical and hazardous wastes. Observe all federal, state, and local environmental regulations. 14 - Transport Information RID/ADR Non-hazardous for road transport. IMDG Non-hazardous for sea transport. IATA Non-hazardous for air transport. 15 - Regulatory Information CLASSIFICATION AND LABELING ACCORDING TO EU DIRECTIVES INDICATION OF DANGER: Xi Irritant. R-PHRASES: 36/37 Irritating to eyes and respiratory system. S-PHRASES: 26-36 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Wear suitable protective clothing. COUNTRY SPECIFIC INFORMATION Germany WGK: 3 Self-Classification 16 - Other Information WARRANTY The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. Inc., shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. Copyright 2010 Co. License granted to make unlimitedpaper copies for internal use only. DISCLAIMER For R&D use only. Not for drug, household or other uses. ALDRICHwww.molbase.com SECTION 16 - ADDITIONAL INFORMATION N/A |
|
毒理学数据: 急性毒性:大鼠经口LD50:>50 gm/kg;兔子经皮LD50: >20 mL/kg; 生态学数据: 通常对水体是稍微有害的,不要将未稀释或大量产品接触地下水,水道或污水系统,未经政府许可勿将材料排入周围环境。 CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| 个人防护装备 | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| 危害码 (欧洲) | Xi:Irritant |
| 风险声明 (欧洲) | R36/37 |
| 安全声明 (欧洲) | S26-S36 |
| 危险品运输编码 | UN1950 |
| RTECS号 | ED7800000 |
| 危险类别 | 2.1 |
| 上游产品 10 | |
|---|---|
| 下游产品 10 | |