54350-48-0
| 中文名 | 依曲替酯 |
|---|---|
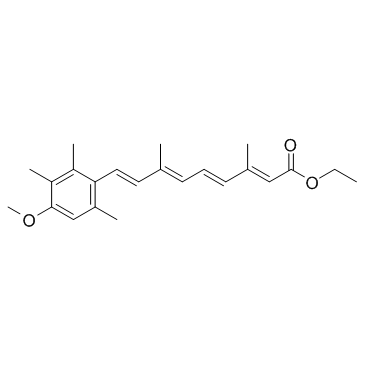
| 英文名 | etretinate |
| 中文别名 |
替维甲
阿维A酯 全反式-9-(4-甲氧基-2,3,6-三甲基苯基)-3,7-二甲基-2,4,6,8-壬四烯酸乙酯 |
| 英文别名 |
ethyl (all-E)-9-(4-methyoxy-2,3,6-trimethylphenyl)-3,7-dimethylnona-2,4,6,8-tetraenoate
2,4,6,8-Nonatetraenoic acid, 9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-, ethyl ester Retinoid 9-(4-methoxy-2,3,6-trimethyl-phenyl)-3,7-dimethyl-nona-2,4,6,8-tetraen-1-oic acid ethyl ester Ethyl etrinoate Ro 10-9359 Etretinatum [INN-Latin] Etretinato ethyl (2E,4E,6E,8E)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethylnona-2,4,6,8-tetraenoate Etretinatum ethyl all-trans-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate EINECS 259-119-3 MFCD00866624 Ethyl 9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate Etretinato [INN-Spanish] Tegison |
| 密度 | 1.0±0.1 g/cm3 |
|---|---|
| 沸点 | 506.4±38.0 °C at 760 mmHg |
| 熔点 | 104-105ºC |
| 分子式 | C23H30O3 |
| 分子量 | 354.483 |
| 闪点 | 219.4±21.4 °C |
| 精确质量 | 354.219482 |
| PSA | 35.53000 |
| LogP | 6.77 |
| 外观性状 | 结晶固体 |
| 蒸汽压 | 0.0±1.3 mmHg at 25°C |
| 折射率 | 1.544 |
| 储存条件 | Amber Vial, -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| 危害码 (欧洲) | Xn |
|---|---|
| 危险品运输编码 | NONH for all modes of transport |
| 上游产品 3 | |
|---|---|
| 下游产品 0 | |