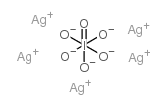
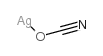
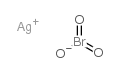
7783-97-3
| 中文名 | 碘酸银 |
|---|---|
| 英文名 | silver,iodate |
| 英文别名 |
silver(1+) iodate
Silver iodate MFCD00014150 Silber(I)-jodat silver(1+) ion iodate EINECS 232-039-6 silver(I) iodate |
| 密度 | 5.525 g/mL at 25 °C(lit.) |
|---|---|
| 熔点 | >200°C (dec.) |
| 分子式 | AgIO3 |
| 分子量 | 282.77100 |
| 精确质量 | 281.79400 |
| PSA | 57.20000 |
| LogP | 0.52930 |
| 外观性状 | 灰白色粉末 |
| 储存条件 | 储存注意事项储存于阴凉、通风的库房。远离火种、热源。库温不超过30℃,相对湿度不超过80%。避免光照。包装密封。应与易(可)燃物、还原剂、活性金属粉末等分开存放,切忌混储。储区应备有合适的材料收容泄漏物。 |
| 稳定性 | 1.溶解度0.0505g/L水溶液(25℃),溶于约1000份35% HNO3(25℃),2.5份10%氨水。 2.稳定性 稳定 3.禁配物 易燃或可燃物、强还原剂、硫、磷、活性金属粉末 4.避免接触的条件 受热、光照 5.聚合危害 不聚合 6.分解产物 碘化物、氧化银 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:0 3.氢键受体数量:3 4.可旋转化学键数量:0 5.互变异构体数量:无 6.拓扑分子极性表面积57.2 7.重原子数量:5 8.表面电荷:0 9.复杂度:36.5 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:2 |
| 更多 | 1.性状:白色棱形结晶或粉末。 2.熔点(℃):>200 3.相对密度(水=1):5.52 4.溶解性:微溶于水,溶于硝酸、氨水。 |
|
Section 1. Chemical Product and Company Identification Silver iodateCatalog Common Name/ Number(s). Trade Name CAS#7783-97-3 Manufacturer
RTECSNot available. SPECTRUM QUALITY PRODUCTS INC. TSCATSCA 8(b) inventory: Silver iodate Commercial Name(s)Not available. CI# Not available. SynonymNot available. IN CASE OF EMERGENCY Silver Iodate Chemical Name Chemical FamilyNot available.CALL (310) 516-8000 Ag-I-O3 Chemical Formula SPECTRUM QUALITY PRODUCTS INC. Section 2.Composition and Information on Ingredients Exposure Limits TWA (mg/m3)STEL (mg/m3) CEIL (mg/m3) NameCAS #% by Weight 1) Silver iodate7783-97-30.01 0.03 100 Toxicological DataSilver iodate on IngredientsLD50: Not available. LC50: Not available. Section 3. Hazards Identification Potential Acute Health Effects Hazardous in case of skin contact (irritant), of eye contact (irritant), of ingestion, of inhalation (lung irritant). Prolonged exposure may result in skin burns and ulcerations. Over-exposure by inhalation may cause respiratory irritation. CARCINOGENIC EFFECTS: Not available. Potential Chronic Health EffectsMUTAGENIC EFFECTS: Not available. TERATOGENIC EFFECTS: Not available. DEVELOPMENTAL TOXICITY: Not available. Repeated or prolonged exposure is not known to aggravate medical condition. Silver iodate Section 4. First Aid Measures Eye ContactCheck for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Cold water may be used. WARM water MUST be used. Get medical attention. Skin ContactIn case of contact, immediately flush skin with plenty of water. Cover the irritated skin with an emollient. Remove contaminated clothing and shoes. Wash clothing before reuse. Thoroughly clean shoes before reuse. Get medical attention. Serious Skin ContactWash with a disinfectant soap and cover the contaminated skin with an anti-bacterial cream. Seek medical attention. InhalationIf inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention. Serious InhalationEvacuate the victim to a safe area as soon as possible. Loosen tight clothing such as a collar, tie, belt or waistband. If breathing is difficult, administer oxygen. If the victim is not breathing, perform mouth-to-mouth resuscitation. Seek medical attention. IngestionDo NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. If large quantities of this material are swallowed, call a physician immediately. Loosen tight clothing such as a collar, tie, belt or waistband. Serious IngestionNot available. Section 5. Fire and Explosion Data Flammability of the Product May be combustible at high temperature. Auto-Ignition Temperature Not available. Flash PointsNot available. Flammable LimitsNot available. Products of CombustionSome metallic oxides. Fire Hazards in Presence of Not available. Various Substances Explosion Hazards in Presence Risks of explosion of the product in presence of mechanical impact: Not available. Risks of explosion of the product in presence of static discharge: Not available. of Various Substances Oxidizing material. Fire Fighting Media and InstructionsDo not use water jet. Use flooding quantities of water. Avoid contact with organic materials. Special Remarks onNot available. Fire Hazards Special Remarks on Explosion Not available. Hazards Section 6. Accidental Release Measures Small SpillUse appropriate tools to put the spilled solid in a convenient waste disposal container. Large Spill Oxidizing material. Stop leak if without risk. Avoid contact with a combustible material (wood, paper, oil, clothing...). Keep substance damp using water spray. Do not touch spilled material. Prevent entry into sewers, basements or confined areas; present at a concentration level above TLV. Check TLV on the MSDS and with local authorities. Silver iodate Section 7. Handling and Storage PrecautionsKeep away from heat. Keep away from sources of ignition. Keep away from combustible material.. Empty containers pose a fire risk, evaporate the residue under a fume hood. Ground all equipment containing material. Do not breathe dust. Wear suitable protective clothing. In case of insufficient ventilation, wear suitable respiratory equipment. If you feel unwell, seek medical attention and show the label when possible. Avoid contact with skin and eyes. StorageKeep container tightly closed. Keep container in a cool, well-ventilated area. Separate from acids, alkalies, reducing agents and combustibles. See NFPA 43A, Code for the Storage of Liquid and Solid Oxidizers. Section 8. Exposure Controls/Personal Protection Engineering ControlsUse process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal ProtectionSplash goggles. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves. Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used a Large Spillto avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product. Exposure LimitsTWA: 0.01 CEIL: 0.03 Consult local authorities for acceptable exposure limits. Section 9. Physical and Chemical Properties Physical state and appearance Solid.OdorNot available. TasteNot available. 282.77 g/mole Molecular Weight ColorNot available. pH (1% soln/water)Not available. Not available. Boiling Point Melting PointDecomposes. Not available. Critical Temperature Specific Gravity5.53 (Water = 1) Vapor PressureNot applicable. Vapor DensityNot available. VolatilityNot available. Odor ThresholdNot available. Water/Oil Dist. Coeff.Not available. Not available. Ionicity (in Water) Dispersion PropertiesNot available. SolubilityVery slightly soluble in cold water. Silver iodate Section 10. Stability and Reactivity Data The product is stable. Stability Instability TemperatureNot available. Not available. Conditions of Instability Not available. Incompatibility with various substances CorrosivityNon-corrosive in presence of glass. Special Remarks onNot available. Reactivity Special Remarks onNot available. Corrosivity PolymerizationWill not occur. Section 11. Toxicological Information Routes of EntryAbsorbed through skin. Eye contact. Inhalation. Ingestion. Toxicity to AnimalsLD50: Not available. LC50: Not available. Chronic Effects on Humans Not available. Other Toxic Effects onHazardous in case of skin contact (irritant), of ingestion, of inhalation (lung irritant). Humans Special Remarks onNot available. Toxicity to Animals Special Remarks onNot available. Chronic Effects on Humans Special Remarks on otherNot available. Toxic Effects on Humans Section 12. Ecological Information EcotoxicityNot available. BOD5 and CODNot available. Products of BiodegradationPossibly hazardous short term degradation products are not likely. However, long term degradation products may arise. The products of degradation are more toxic. Toxicity of the Products of Biodegradation Special Remarks on theNot available. Products of Biodegradation Silver iodate Section 13. Disposal Considerations Waste Disposal Section 14. Transport Information DOT ClassificationCLASS 5.1: Oxidizing material. : Oxidizing Solid, n.o.s. (Silver iodate) UNNA: UN1479 PG: III Identification Not available. Special Provisions for Transport DOT (Pictograms) OXIDIZER 5.1 Section 15. Other Regulatory Information and Pictograms TSCA 8(b) inventory: Silver iodate Federal and State Regulations California Proposition 65 Warnings Other RegulationsOSHA: Hazardous by definition of Hazard Communication Standard (29 CFR 1910.1200). EINECS: This product is on the European Inventory of Existing Commercial Chemical Substances. WHMIS (Canada) CLASS C: Oxidizing material. Other Classifications DSCL (EEC)R8- Contact with combustible material may cause fire. R36/37/38- Irritating to eyes, respiratory system and skin. Health Hazard HMIS (U.S.A.)2 National Fire Protection 1 Flammability 1 Association (U.S.A.) Fire Hazard 2 0 Reactivity Health Reactivity 0 Specific hazard Personal Protection E WHMIS (Canada) (Pictograms) DSCL (Europe) (Pictograms) TDG (Canada) (Pictograms) 5.1 Silver iodate ADR (Europe) (Pictograms) Protective Equipment Gloves. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Wear appropriate respirator when ventilation is inadequate. SECTION 16 - ADDITIONAL INFORMATION N/A |
|
毒理学数据: 1.急性毒性 暂无资料 2.刺激性 暂无资料 3.致畸性 频繁使用碘化物可致胎儿死亡,严重的甲状腺肿和甲状腺机能衰退,新生儿呈现克汀病样体征。 生态学数据: 1.生态毒性 暂无资料 2.生物降解性 暂无资料 3.非生物降解性 暂无资料
|
| 危害码 (欧洲) | O,Xi |
|---|---|
| 风险声明 (欧洲) | R8 |
| 安全声明 (欧洲) | S17-S26-S36/37/39 |
| 危险品运输编码 | UN 1479 5.1/PG 2 |
| WGK德国 | 3 |
| 包装等级 | II |
| 危险类别 | 5.1 |
|
~99% 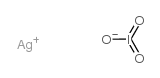
7783-97-3 |
| 文献:Gmelin Handbook: Ag: MVol.B2, 8.32.1, page 446 - 448 |
|
~% 
7783-97-3 |
| 文献:Journal of the American Chemical Society, , vol. 25, p. 637 - 641 Ag: MVol.B2, 8.25.1, page 434 - 436 |
|
~% 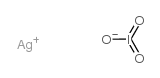
7783-97-3 |
| 文献:Inorganic Chemistry, , vol. 4, p. 257 - 259 |
|
~% 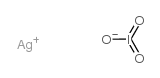
7783-97-3 |
| 文献:Mikrochemie, , vol. 6, p. 82 - 91 Ag: MVol.B2, 8.25.1, page 434 - 436 |
|
~% 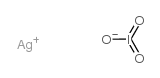
7783-97-3 |
| 文献:Ann. Chim. Phys., , vol. 21, p. 28 - 28 |
|
~% 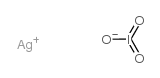
7783-97-3 |
| 文献:Journal of the Chemical Society, , vol. 101, p. 1852 - 1866 C: MVol.D3, 6.2.6.6.8, page 261 - 263 |
|
~% 
7783-97-3 |
| 文献:Anales Asoc. Quim. Arg., , vol. 21, p. 37 - 46 C.I, , p. 1033 C: MVol.D3, 6.2.6.6.8, page 261 - 263 |
|
~% 
7783-97-3 |
| 文献:Ber. Dtsch. Chem. Ges., , vol. 27, p. 502 - 509 Ag: MVol.B2, 8.25.1, page 434 - 436 |
|
~% 
7783-97-3 |
| 文献:Ber. Dtsch. Chem. Ges., , vol. 27, p. 502 - 509 Ag: MVol.B2, 8.25.1, page 434 - 436 |
| 上游产品 9 | |
|---|---|
| 下游产品 9 | |