114676-59-4
| 中文名 | (4R)-4-羟基-D-脯氨酸甲酯盐酸盐 |
|---|---|
| 英文名 | D-Proline, 4-hydroxy-, methyl ester, (Hydrochloride) (1:1), (4R) |
| 中文别名 | 顺式-4-羟基-D-脯氨酸甲酯盐酸盐 |
| 英文别名 |
D-Proline, 4-hydroxy-, methyl ester, (4R)-, hydrochloride (1:1)
Trans-4-hydroxy-L-proline methyl ester hydrochloride Methyl (4R)-4-hydroxy-L-prolinate hydrochloride (1:1) Methyl (4R)-4-hydroxy-D-prolinate hydrochloride (1:1) CIS-4-HYDROXY-D-PROLINE METHYL ESTER L-Proline, 4-hydroxy-, methyl ester, (4R)-, hydrochloride (1:1) cis-4-hydroxy-D-proline methyl ester hydrochloride methyl cis 4-hydroxyl-D-proline (2R,4R)-4-Hydroxypyrrolidine-2-carboxylic acid methyl ester hydrochloride (2R,4R)-Methyl 4-hydroxypyrrolidine-2-carboxylate hydrochloride |
| 描述 | D-脯氨酸,4-羟基-甲酯盐酸盐是一种不可切割的ADC连接物,用于合成抗体药物结合物(ADC)。D-脯氨酸,4-羟基-甲酯盐酸盐也是一种基于烷基链的PROTAC连接剂,可用于合成PROTAC[1][2] |
|---|---|
| 相关类别 | |
| 靶点 |
Non-cleavable |
| 体外研究 | ADC由抗体组成,抗体通过ADC连接体连接ADC细胞毒素[1]。PROTAC包含两个不同的配体,通过连接体连接;一个是E3泛素连接酶的配体,另一个是靶蛋白的配体。PROTAC利用细胞内泛素-蛋白酶体系统选择性降解目标蛋白质[2]。 |
| 参考文献 |
| 熔点 | 121-123 ºC |
|---|---|
| 分子式 | C6H12ClNO3 |
| 分子量 | 181.617 |
| 精确质量 | 181.050568 |
| PSA | 58.56000 |
| LogP | 0.01300 |
| 储存条件 | 室温 |
|
~99% 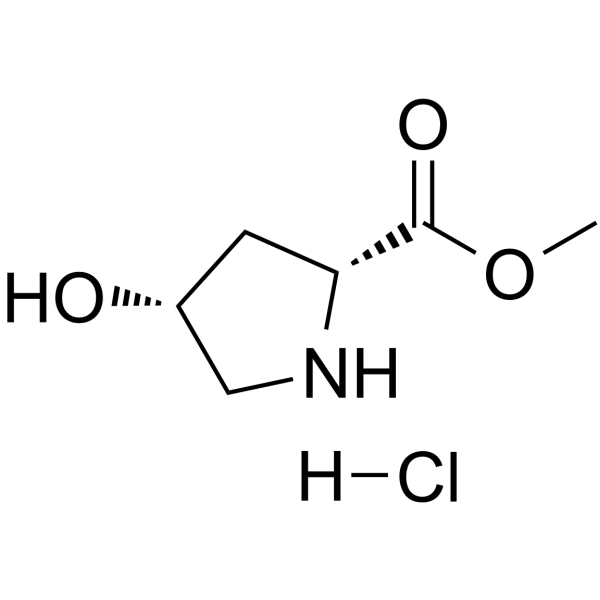
114676-59-4 |
| 文献:VICURON PHARMACEUTICALS, INC. Patent: WO2004/7444 A2, 2004 ; Location in patent: Page 135 ; |
|
~91% 
114676-59-4 |
| 文献:Peng, Jianbiao; Clive, Derrick L. J. Journal of Organic Chemistry, 2009 , vol. 74, # 2 p. 513 - 519 |
|
~99% 
114676-59-4 |
| 文献:Shionogi Seiyaku Kabushiki Kaisha Patent: US5317016 A1, 1994 ; |
|
~98% 
114676-59-4 |
| 文献:Rosen; Fesik; Chu; Pernet Synthesis, 1988 , # 1 p. 40 - 44 |
|
~% 
114676-59-4 |
| 文献:WO2005/108358 A2, ; Page/Page column 35 ; WO 2005/108358 A2 |
|
~% 
114676-59-4 |
| 文献:WO2014/32 A1, ; |
|
~% 
114676-59-4 |
| 文献:WO2014/32 A1, ; |
| 上游产品 5 | |
|---|---|
| 下游产品 4 | |