127-33-3
| 中文名 | 地美环素 |
|---|---|
| 英文名 | demeclocycline |
| 英文别名 |
Ledermycin hydrochloride
7-chloro-6-demethyltetracycline 6-demethyl-7-chlorotetracycline (2E,4S,4aS,5aS,6S,12aS)-2-[Amino(hydroxy)methylene]-7-chloro-4-(dimethylamino)-6,10,11,12a-tetrahydroxy-4a,5a,6,12a-tetrahydrotetracene-1,3,12(2H,4H,5H)-trione Elkamicina DMCTC (4aS)-7-Chlor-4c-dimethylamino-3,6t,10,12,12a-pentahydroxy-1,11-dioxo-(4ar,5ac,12ac)-1,4,4a,5,5a,6,11,12a-octahydro-naphthacen-2-carbonsaeure-amid Declomycin EINECS 204-834-8 Demeclocyclinum 7-chloro-4-dimethylamino-3,6,10,12,12a-pentahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydro-naphthacene-2-carboxylic acid amide (2E,4S,4aS,5aS,6S,12aS)-2-[Amino(hydroxy)methylene]-7-chloro-4-(dimethylamino)-6,10,11,12a-tetrahydroxy-4a,5a,6,12a-tetrahydro-1,3,12(2H,4H,5H)-tetracenetrione Demethylchlortetracycline(DMCT) Demeclociclina Ledermycin domeclocycline (4S,4aS,5aS,6S,12aR)-7-chloro-4-(dimethylamino)-1,6,10,11,12a-pentahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4H-tetracene-2-carboxamide |
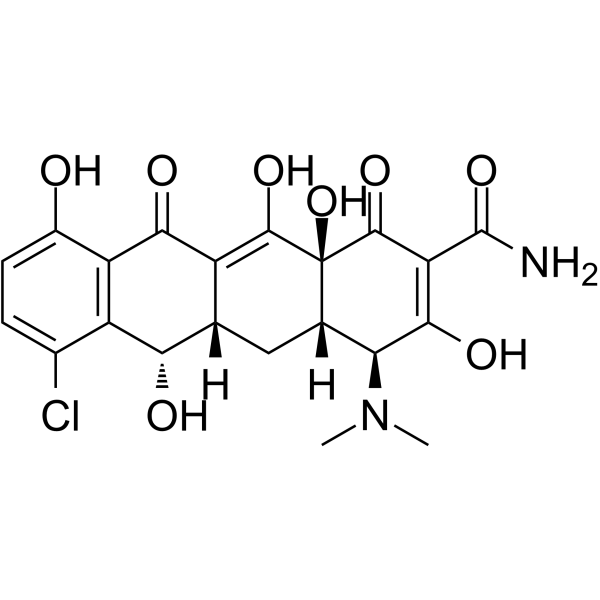
| 描述 | 去甲环素是一种口服活性四环素抗生素。去甲环素通过与30S核糖体亚基结合来抑制氨基酰基tRNA的结合,从而损害蛋白质合成。脱氯环素对多种细菌感染具有抗菌活性[1][2]。 |
|---|---|
| 相关类别 | |
| 体外研究 | 脱氯环素(0-100μM;24小时)处理降低mpkCCD细胞中AQP2的丰度[3]。去甲环素(10μM;24小时)治疗促进单核细胞和巨噬细胞的活性[4]。去甲环素(1-10μM;72小时)治疗直接影响脑肿瘤细胞的生长[4]。Western印迹分析[3]细胞系:MpkCCD细胞浓度:0-100μM孵育时间:24小时结果:MpkCCD细胞中AQP2丰度降低,在50μM时有显著影响。细胞存活率测定[4]细胞系:小鼠骨髓来源的巨噬细胞和单核细胞浓度:10μM孵育时间:24小时结果:增强TNF-α的产生并调节单核细胞功能。细胞存活率测定[4]细胞系:脑肿瘤起始细胞浓度:1、5和10μM孵育时间:72小时结果:通过两种方式抑制细胞生长:使用单核细胞作为中介,并直接影响脑肿瘤起始的细胞增殖和球体形成能力。 |
| 体内研究 | 去甲环素(腹腔注射;40mg/kg;每日一次;48小时)治疗可显著降低低钠血症,显著纠正低渗透压,且无肾毒性[3]。动物模型:低钠血症雄性Wistar大鼠[3]剂量:40mg/kg给药:腹腔注射;40mg/kg;每日一次;48小时结果:尿量增加,尿渗透压降低,并导致水排泄分数显著增加。动物模型:低钠血症雄性Wistar大鼠[3]剂量:40mg/kg给药:腹腔注射;40mg/kg;每日一次;48小时结果:表明肾内髓质对AQP2和AC5/6有特异性作用,而非二次毒性作用。 |
| 参考文献 |
| 密度 | 1.8±0.1 g/cm3 |
|---|---|
| 沸点 | 684.5±55.0 °C at 760 mmHg |
| 分子式 | C21H21ClN2O8 |
| 分子量 | 464.853 |
| 闪点 | 367.8±31.5 °C |
| 精确质量 | 464.098633 |
| PSA | 181.62000 |
| LogP | 0.57 |
| 蒸汽压 | 0.0±2.2 mmHg at 25°C |
| 折射率 | 1.761 |
| 储存条件 | 室温 |
| 分子结构 | 1、 摩尔折射率:109.25 2、 摩尔体积(cm3/mol):265.1 3、等张比容(90.2K):854.8 4、表面张力(dyne/cm):107.9 5、极化率(10-24cm3):43.31 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):0.7 2.氢键供体数量:6 3.氢键受体数量:9 4.可旋转化学键数量:2 5.互变异构体数量:132 6.拓扑分子极性表面积182 7.重原子数量:32 8.表面电荷:0 9.复杂度:961 10.同位素原子数量:0 11.确定原子立构中心数量:5 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| 上游产品 0 | |
|---|---|
| 下游产品 1 | |