149934-19-0
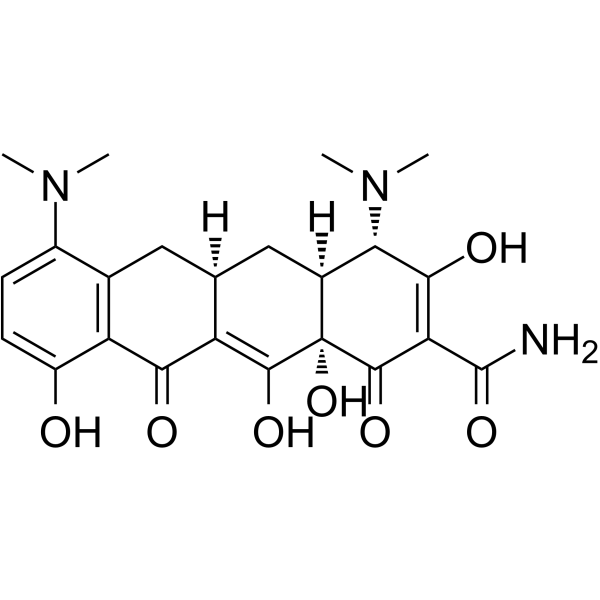
| 英文名 | (4S,4aS,5aR,12aS)-9-Amino-4,7-bis(dimethylamino)-3,10,12,12a-tetr ahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydro-2-tetracenecar boxamide |
|---|---|
| 英文别名 |
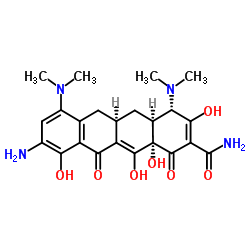
9-Aminominocycline
(4S,4aS,5aR,12aS)-4-(dimethylamino)-3,10,12,12a-tetrahydroxy-7-((methoxymethylamino)methyl)-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide monohydrochloride Sarecycline Hydrochloride Sarecycline Hydrochloride [USAN] (4S,4aS,5aR,12aS)-4-dimethylamino-3,10,12,12a-tetrahydroxy-7-[(methoxy(methyl)amino)-methyl]-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydro-naphthacene-2-carboxylic acid amide mono hydrochloride 9-aminotetracycline (4S,4aS,5aR,12aS)-9-Amino-4,7-bis(dimethylamino)-3,10,12,12a-tetrahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydro-2-tetracenecarboxamide 9-amino-minocycline (4S,4aS,5aR,12S)-9-amino-4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxanaphthacene-2-carboxamide (4S,4aS,5aR,12aS)-9-amino-4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide 2-Naphthacenecarboxamide,4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-7-((methoxymethylamino)methyl)-1,11-dioxo-,hydrochloride (1:1),(4S,4aS,5aR,12aS) |
| 密度 | 1.6±0.1 g/cm3 |
|---|---|
| 沸点 | 830.7±65.0 °C at 760 mmHg |
| 分子式 | C23H28N4O7 |
| 分子量 | 472.491 |
| 闪点 | 456.2±34.3 °C |
| 精确质量 | 472.195801 |
| PSA | 191.64000 |
| LogP | -1.69 |
| 蒸汽压 | 0.0±3.2 mmHg at 25°C |
| 折射率 | 1.737 |
|
~% 
149934-19-0 |
| 文献:WYETH LLC; YANG, ChunHua; DILLON, John Leo Jr.; NAIK, Ramachandra Patent: WO2010/114680 A1, 2010 ; Location in patent: Page/Page column 27-28 ; |
|
~% 
149934-19-0 |
| 文献:Sum, Phaik-Eng; Ross, Adma T.; Petersen, Peter J.; Testa, Raymond T. Bioorganic and Medicinal Chemistry Letters, 2006 , vol. 16, # 2 p. 400 - 403 |