202407-03-2
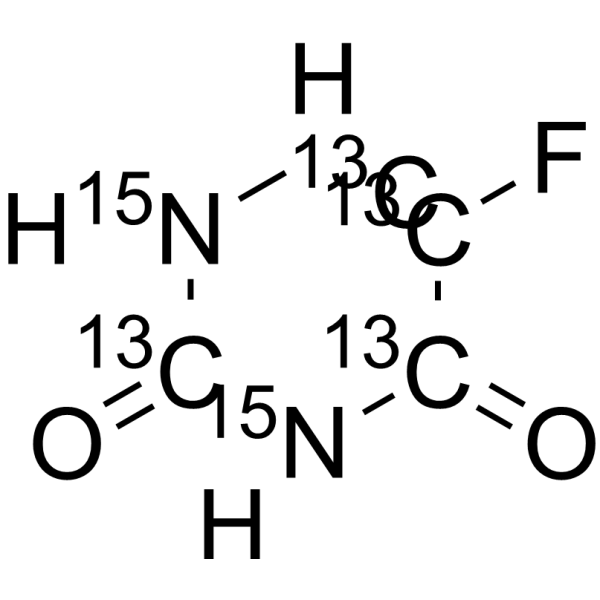
| 中文名 | 5-氟脲嘧啶-13C4,15N2 |
|---|---|
| 英文名 | 5-Fluorouracil-13C4,15N2 |
| 描述 | 5-氟尿嘧啶-13C4,15N2是13C和15N标记的5-氟尿嘧啶[1]。5-氟尿嘧啶(5-FU)是尿嘧啶的类似物,是一种有效的抗肿瘤药物。5-氟尿嘧啶通过抑制胸苷酸合成酶来影响嘧啶的合成,从而耗尽细胞内的dTTP池。5-氟尿嘧啶可诱导细胞凋亡,可作为化学增敏剂[2][3]。5-氟尿嘧啶也能抑制艾滋病病毒[4]。 |
|---|---|
| 相关类别 | |
| 体外研究 | 氢、碳和其他元素的稳定重同位素已被掺入药物分子中,主要作为药物开发过程中定量的示踪剂。头韵因其可能影响药物的药代动力学和代谢特征而受到关注[1]。 |
| 参考文献 |
| 分子式 | 13C4H3F15N2O2 |
|---|---|
| 分子量 | 136.03 |