20830-81-3
| 中文名 | 道诺霉素 |
|---|---|
| 英文名 | daunorubicin |
| 中文别名 |
柔红霉素
红保霉素 |
| 英文别名 |
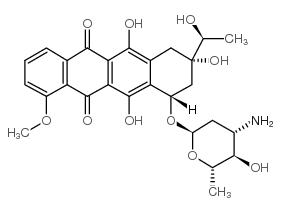
(1S,3S)-3-acetyl-3,5,12-trihydroxy-10-(methyloxy)-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranoside
DAUNOMYCIN daunamycin (1S,3S)-3-Acetyl-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydro-1-tetracenyl 3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranoside (8S-cis)-8-Acetyl-10-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro--6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione daunoxome (8S-cis)-8-Acetyl-10-[(3-amino-2,3,6-trideoxy-a-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione fi6339 cerubidin rubomycinc Daunorubicin (8S,10S)-8-Acetyl-10-[(3-amino-2,3,6-trideoxy-a-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione (8S,10S)-8-Acetyl-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-6,8,11-trihydroxy-1-methoxy-7,8,9,10-tetrahydrotetracen-5,12-dion (8S,10S)-8-acétyl-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-méthyltétrahydro-2H-pyran-2-yl]oxy}-6,8,11-trihydroxy-1-méthoxy-7,8,9,10-tétrahydrotétracène-5,12-dione EINECS 245-723-4 (8S,10S)-8-acetyl-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-6,8,11-trihydroxy-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione Cerubidine(R) (1S,3S)-3-Acetyl-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranoside (8S,10S)-8-Acetyl-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-6,8,11-trihydroxy-1-methoxy-7,8,9,10-tetrahydro-5,12-tetracenedione daunorubicinum [INN_la] RUBIDOMYCIN |
| 描述 | Daunorubicin(RP13057)能抑制DNA和RNA合成,对DNA合成的Ki为0.02 μM。 |
|---|---|
| 相关类别 | |
| 靶点 |
Topoisomerase II |
| 体外研究 | Molt-4细胞中柔红霉素(Dnr)的平均IC50值为0.04μM。柔红霉素属于蒽环霉素,一组细胞毒性化学治疗剂。蒽环霉素的细胞毒性作用是由DNA插入和通过抑制拓扑异构酶II以及产生活性氧来干扰DNA转录和复制的能力引起的[2]柔红霉素抑制HeLa细胞中DNA和RNA合成的浓度范围0.2至2μM。对于人胰腺细胞系L3.6中的柔红霉素(Dnr),IC50值为0.4μM[3]。 |
| 体内研究 | 与对照组相比,柔红霉素组(3mg/kg,iv)中尿蛋白排泄,血清肌酐和血尿素氮(BUN)水平显着增加。与对照组相比,柔红霉素(DNR)的施用导致肾组织中丙二醛(MDA)水平显着增加[4]。 |
| 细胞实验 | 使用MTT测定评估对柔红霉素的化学敏感性。简而言之,96孔板设置有初始密度为2×105个细胞/ mL的细胞,并在不存在和存在9种不同浓度的不同浓度的存在下,在5%CO 2的气氛中在37℃下孵育72小时。柔红霉素(Dnr)或Dox的范围为1.90至0.007μM,一式三份。温育后,向每个孔中加入10μLMTT溶液(5mg / mL四唑盐),并将板在37℃下再温育4小时。通过在10mM HCl溶液中加入100μL10%SDS溶解甲crystals盐晶体并在37℃下温育过夜。通过96孔酶联免疫吸附测定(ELISA)读板仪在540nm处用参比在650nm处测量吸光度。化学敏感性表示为IC 50,其是与没有药物生长的对照细胞相比引起50%细胞存活的药物浓度。使用Microsoft Excel [2]进行计算。 |
| 动物实验 | 大鼠[4]使用8周龄雄性Sprague-Dawley大鼠。在开始实验之前,将动物隔离并使其适应另外2周。在第0天,每只动物以3mg / kg的剂量接受单次静脉内注射柔红霉素(iv)。柔红霉素以48小时的间隔以三次相等的注射给药,持续一周,以达到9mg / kg的累积剂量,这充分证明产生心脏毒性和肾毒性。向年龄匹配的大鼠注射相应体积的0.9%NaCl并用作对照(组对照; n = 5)。将22只DNR处理的大鼠随机分成两组,口服替米沙坦(10mg / kg /天;柔红霉素+替米沙坦组; n = 10)或载体(柔红霉素组; n = 12)。替米沙坦的剂量是根据之前的报告选择的。替米沙坦的给药在与柔红霉素给药的同一天开始,并在停止使用柔红霉素后持续另外5周(总共6周)。该研究期限是根据以前的报告选择的。在第41天,将大鼠单独置于代谢笼中以进行24小时尿液收集以测量蛋白质浓度并测量体重(BW)。在研究期结束后(6周),处死大鼠并收获肾组织用于半定量免疫印迹和免疫组织化学研究。 |
| 参考文献 |
| 密度 | 1.6±0.1 g/cm3 |
|---|---|
| 沸点 | 770.0±60.0 °C at 760 mmHg |
| 熔点 | 155ºC |
| 分子式 | C27H29NO10 |
| 分子量 | 527.520 |
| 闪点 | 419.5±32.9 °C |
| 精确质量 | 527.179138 |
| PSA | 185.84000 |
| LogP | 2.92 |
| 外观性状 | 橙色-红色粉末 |
| 蒸汽压 | 0.0±2.8 mmHg at 25°C |
| 折射率 | 1.692 |
| 储存条件 | 放入紧密的贮藏器内 |
| 稳定性 | 避免接触强氧化物 |
| 分子结构 | 1、 摩尔折射率:129.98 2、 摩尔体积(m3/mol):339.4 3、 等张比容(90.2K):1037.9 4、 表面张力(dyne/cm):87.4 5、 极化率(10-24cm3):51.52 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:5 3.氢键受体数量:11 4.可旋转化学键数量:4 5.互变异构体数量:54 6.拓扑分子极性表面积186 7.重原子数量:38 8.表面电荷:0 9.复杂度:960 10.同位素原子数量:0 11.确定原子立构中心数量:6 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1.性状:未确定 2.密度(g/mL,25/4℃):未确定 3.相对蒸汽密度(g/mL,空气=1):未确定 4.熔点(ºC):未确定 5.沸点(ºC,常压):未确定 6.沸点(ºC,5.2kPa):未确定 7.折射率:未确定 8.闪点(ºC):未确定 9.比旋光度(º):未确定 10.自燃点或引燃温度(ºC):未确定 11.蒸气压(kPa,25ºC):未确定 12.饱和蒸气压(kPa,60ºC):未确定 13.燃烧热(KJ/mol):未确定 14.临界温度(ºC):未确定 15.临界压力(KPa):未确定 16.油水(辛醇/水)分配系数的对数值:未确定 17.爆炸上限(%,V/V):未确定 18.爆炸下限(%,V/V):未确定 19.溶解性:未确定 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| 风险声明 (欧洲) | R3249 |
|---|---|
| 危险品运输编码 | UN 3249 |
| 包装等级 | III |
| 危险类别 | 6.1(b) |
|
~% 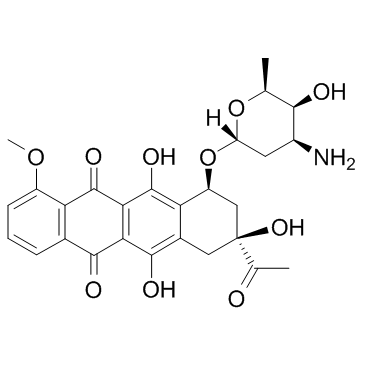
20830-81-3 |
| 文献:EP2660255 A1, ; Page/Page column ; |
|
~% 
20830-81-3
详细
|
| 文献:Journal of the American Chemical Society, , vol. 113, # 1 p. 308 - 315 |
|
~% 
20830-81-3 |
| 文献:Journal of Medicinal Chemistry, , vol. 23, # 11 p. 1166 - 1170 |
|
~% 
20830-81-3 |
| 文献:Bioorganic and Medicinal Chemistry, , vol. 7, # 8 p. 1597 - 1610 |
|
~% 
20830-81-3 |
| 文献:Bioorganic and Medicinal Chemistry, , vol. 7, # 8 p. 1597 - 1610 |
|
~% 
20830-81-3 |
| 文献:Tetrahedron Letters, , vol. 41, # 25 p. 4871 - 4874 |
| 上游产品 3 | |
|---|---|
| 下游产品 8 | |


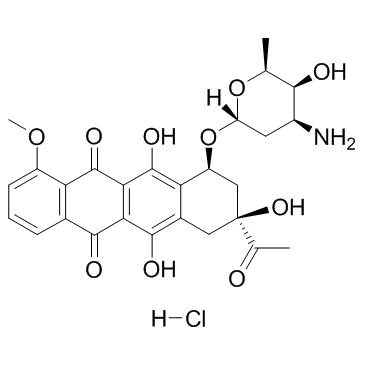
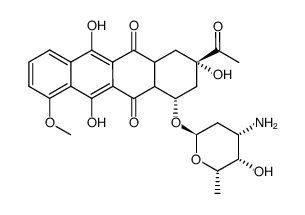
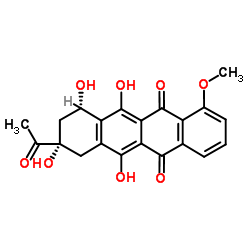




![N-[6-[(3-acetyl-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-2,4-dihydro-1H-tetracen-1-yl)oxy]-3-hydroxy-2-methyloxan-4-yl]acetamide结构式](https://image.chemsrc.com/caspic/437/32385-10-7.png)


