rac Fesoterodine-d14 fumarate
更新时间:2025-08-26 19:53:54
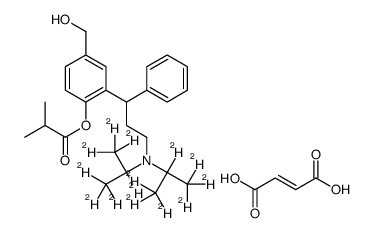
rac Fesoterodine-d14 fumarate结构式

|
常用名 | rac Fesoterodine-d14 fumarate | 英文名 | rac Fesoterodine-d14 Fumarate |
|---|---|---|---|---|
| CAS号 | 1185237-08-4 | 分子量 | 541.73500 | |
| 密度 | N/A | 沸点 | N/A | |
| 分子式 | C30H27D14NO7 | 熔点 | N/A | |
| MSDS | N/A | 闪点 | N/A |
rac Fesoterodine-d14 fumarate用途(Rac)-非索替罗定-d14富马酸盐是一种标记的外消旋非索替罗定。非索替罗定是一种口服活性、非亚型选择性、竞争性毒蕈碱受体(mAChR)拮抗剂,M1、M2、M3、M4、M5受体的pKi值分别为8.0、7.7、7.4、7.3、7.5。非索替罗定用于膀胱过度活动症(OAB)[1][2]。 |
| 英文名 | rac Fesoterodine-d14 Fumarate |
|---|---|
| 英文别名 | 更多 |
| 描述 | (Rac)-非索替罗定-d14富马酸盐是一种标记的外消旋非索替罗定。非索替罗定是一种口服活性、非亚型选择性、竞争性毒蕈碱受体(mAChR)拮抗剂,M1、M2、M3、M4、M5受体的pKi值分别为8.0、7.7、7.4、7.3、7.5。非索替罗定用于膀胱过度活动症(OAB)[1][2]。 |
|---|---|
| 相关类别 | |
| 体外研究 | 氢、碳和其他元素的稳定重同位素已被纳入药物分子,主要作为药物开发过程中定量的示踪剂。氘化因其可能影响药物的药代动力学和代谢特征而受到关注[1]。 |
| 参考文献 |
| 分子式 | C30H27D14NO7 |
|---|---|
| 分子量 | 541.73500 |
| 精确质量 | 541.37600 |
| PSA | 124.37000 |
| LogP | 5.09290 |
| InChIKey | MWHXMIASLKXGBU-VCQPCXMRSA-N |
| SMILES | CC(C)C(=O)Oc1ccc(CO)cc1C(CCN(C(C)C)C(C)C)c1ccccc1.O=C(O)C=CC(=O)O |
| [2-[3-[bis(1,1,1,2,3,3,3-heptadeuteriopropan-2-yl)amino]-1-phenylpropyl]-4-(hydroxymethyl)phenyl] 2-methylpropanoate,(E)-but-2-enedioic acid |

