RecA-mediated strand invasion of DNA by oligonucleotides substituted with 2-aminoadenine and 2-thiothymine.
Georges Lahoud, Khalil Arar, Ya-Ming Hou, Howard Gamper
文献索引:Nucleic Acids Res. 36(21) , 6806-15, (2008)
全文:HTML全文
摘要
Sequence-specific recognition of DNA is a critical step in gene targeting. Here we describe unique oligonucleotide (ON) hybrids that can stably pair to both strands of a linear DNA target in a RecA-dependent reaction with ATP or ATPgammaS. One strand of the hybrids is a 30-mer DNA ON that contains a 15-nt-long A/T-rich central core. The core sequence, which is substituted with 2-aminoadenine and 2-thiothymine, is weakly hybridized to complementary locked nucleic acid or 2'-OMe RNA ONs that are also substituted with the same base analogs. Robust targeting reactions took place in the presence of ATPgammaS and generated metastable double D-loop joints. Since the hybrids had pseudocomplementary character, the component ONs hybridized less strongly to each other than to complementary target DNA sequences composed of regular bases. This difference in pairing strength promoted the formation of joints capable of accommodating a single mismatch. If similar joints can form in vivo, virtually any A/T-rich site in genomic DNA could be selectively targeted. By designing the constructs so that the DNA ON is mismatched to its complementary sequence in DNA, joint formation might allow the ON to function as a template for targeted point mutation and gene correction.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
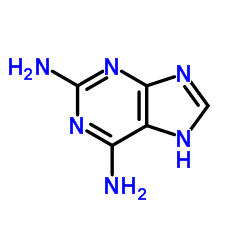 |
2,6-二氨基嘌呤
CAS:1904-98-9 |
C5H6N6 |
|
Effect of substituents on the excited-state dynamics of the ...
2010-01-01 [Phys. Chem. Chem. Phys. 12(20) , 5375-88, (2010)] |
|
Formation and helicity control of ssDNA templated porphyrin ...
2013-02-01 [Chem. Commun. (Camb.) 49(10) , 1020-2, (2013)] |
|
Simulations of A-RNA duplexes. The effect of sequence, solut...
2012-08-23 [J. Phys. Chem. B 116(33) , 9899-916, (2012)] |
|
Biotransformation of 2,6-diaminopurine nucleosides by immobi...
2012-01-01 [Biotechnol. Prog. 28(5) , 1251-6, (2012)] |
|
Recombination R-triplex: H-bonds contribution to stability a...
2006-01-01 [Nucleic Acids Res. 34(11) , 3239-45, (2006)] |