Preparation of the 2'-deoxynucleosides of 2,6-diaminopurine and isoguanine by direct glycosylation.
Joseph W Arico, Amy K Calhoun, Larry W McLaughlin
文献索引:J. Org. Chem. 75(5) , 1360-5, (2010)
全文:HTML全文
摘要
The purine nucleoside 2,6-diaminopurine-2'-deoxyriboside is prepared by the direct glycosylation of the 2,6-bis(tetramethylsuccinimide) derivative of the parent purine heterocycle 4 with 2-deoxy-3,5-di-O-(p-toluoyl)-alpha-D-erythro-pentofuranosyl chloride 5 using the sodium salt method. 2'-Deoxyisoguanosine is prepared from 2,6-diaminopurine by a five-step procedure. The purine heterocycle isoguanine is prepared by selective diazotization of 2,6-diaminopurine and then converted to the N9-trityl derivative to increase solubility. After silylation of the O(2)-carbonyl with TMSCl, the N(6)-amino group is protected as the tetramethylsuccinimide (M(4)SI). The O(2)-carbonyl is protected as the DPC derivative, and the trityl group is removed. The resulting product is glycosylated in good yield to generate fully protected 2'-deoxyisoguanosine.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
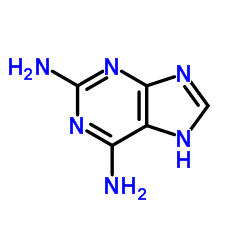 |
2,6-二氨基嘌呤
CAS:1904-98-9 |
C5H6N6 |
|
Effect of substituents on the excited-state dynamics of the ...
2010-01-01 [Phys. Chem. Chem. Phys. 12(20) , 5375-88, (2010)] |
|
Formation and helicity control of ssDNA templated porphyrin ...
2013-02-01 [Chem. Commun. (Camb.) 49(10) , 1020-2, (2013)] |
|
Simulations of A-RNA duplexes. The effect of sequence, solut...
2012-08-23 [J. Phys. Chem. B 116(33) , 9899-916, (2012)] |
|
Biotransformation of 2,6-diaminopurine nucleosides by immobi...
2012-01-01 [Biotechnol. Prog. 28(5) , 1251-6, (2012)] |
|
Recombination R-triplex: H-bonds contribution to stability a...
2006-01-01 [Nucleic Acids Res. 34(11) , 3239-45, (2006)] |