[14C]leucine chloromethylketone interaction with sarcoma 37 cell plasma membrane components.
R H Matthews, G E Milo, T L McMichael, N J Lewis
文献索引:Cancer Lett. 16(2) , 207-18, (1982)
全文:HTML全文
摘要
Leucine chloromethylketone labeling of viable S37 cells was preferential for the plasma membrane fraction. The pattern of radiolabeling of the plasma membrane proteins was time dependent. After 5 min the radiolabel was localized with glutamyl transpeptidase, and subsequently with other physiologically active proteins as a function of time after incubation. Labeling of proteins was temperature dependent and incubation of viable S37 cells with the radiolabeled substrate at 0 degrees C yielded little or no radioactivity localized in the plasma membrane. The molecular weight of one radiolabeled substrate--membrane protein complex was estimated on sodium dodecyl sulfate polyacrylamide gel electrophoresis to be between 100,000-200,000.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
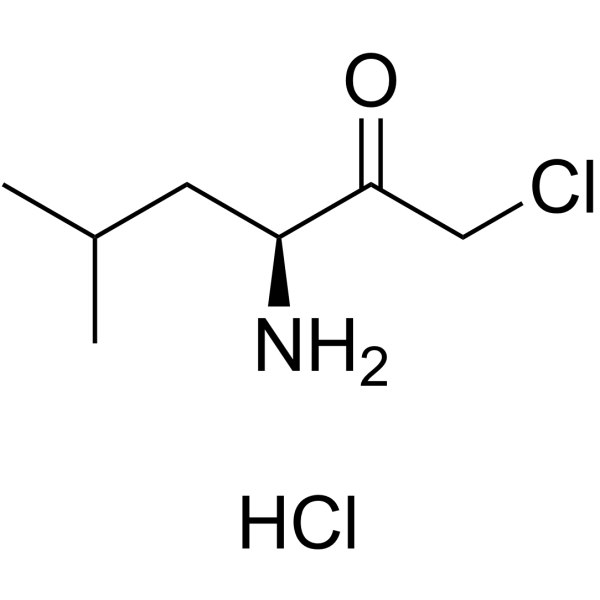 |
H-Leu-CMK.HCl
CAS:54518-92-2 |
C7H15Cl2NO |
|
Acylpeptide hydrolase: inhibitors and some active site resid...
1992-02-25 [J. Biol. Chem. 267 , 3811-3818, (1992)] |
|
Acetylleucine chloromethyl ketone, an inhibitor of acylpepti...
1999-09-16 [Biochem. Biophys. Res. Commun. 263 , 139-142, (1999)] |
|
Chloromethyl ketones are insulin-like stimulators of lipid s...
1998-01-01 [Arch. Insect Biochem. Physiol. 39 , 9-17, (1998)] |
|
Metabolism and degradation products of recombinant human ins...
1999-03-01 [Xenobiotica 29 , 281-295, (1999)] |
|
Inhibition of S37 ascites cell amino acid transport systems ...
1980-10-02 [Biochim. Biophys. Acta 601(3) , 640-53, (1980)] |