Determination of the intrinsic Michaelis constant of immobilized alpha-chymotrypsin.
S Blais, R Lortie
文献索引:J. Biol. Chem. 268(25) , 18637-9, (1993)
全文:HTML全文
摘要
The Michaelis constant of alpha-chymotrypsin, immobilized on a glutaraldehyde-activated silicate support, for N-glutaryl-L-phenylalanine-p-nitroanilide was determined and was found to be identical with that of the enzyme in solution. The influence of intraparticular diffusion was taken into account by immobilizing different amounts of enzyme, thus changing the magnitude of diffusional constraints and extrapolating apparent Michaelis constants, determined for each amount of immobilized enzyme, to zero diffusional constraints. The possible effect of the immobilized enzyme distribution inside the porous matrix was investigated through numerical simulations.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
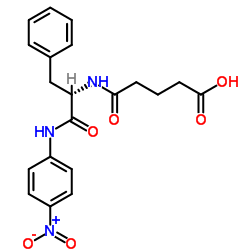 |
戊二酰-L-苯丙氨酰-对硝基苯胺
CAS:5800-34-0 |
C20H21N3O6 |
|
Effect of human serum albumin on the kinetics of N-glutaryl-...
[Protein J. 30 , 143-7, (2011)] |
|
[Kinetic regularities of S2'-stimulation of alpha-chymotryps...
1996-01-01 [Ukr. Biokhim. Zh. 68(3) , 36-41, (1996)] |
|
Occurrence and identity of proteolytic bacteria in adult per...
1994-09-01 [J. Periodont. Res. 29(5) , 365-70, (1994)] |
|
Immobilization of enzymes on a microchannel surface through ...
2005-10-28 [Chem. Commun. (Camb.) (40) , 5062-4, (2005)] |
|
Cell-permeable GPNA with appropriate backbone stereochemistr...
2005-01-14 [Chem. Commun. (Camb.) (2) , 244-6, (2005)] |