| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
α-糜蛋白酶
CAS:9004-07-3 |
|
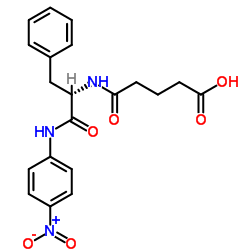 |
戊二酰-L-苯丙氨酰-对硝基苯胺
CAS:5800-34-0 |