Journal of the American Chemical Society
2002-11-13
Highly enantioselective Ag(i)-catalyzed [3 + 2] cycloaddition of azomethine ylides.
James M Longmire, Bin Wang, Xumu Zhang
文献索引:J. Am. Chem. Soc. 124(45) , 13400-1, (2002)
全文:HTML全文
摘要
A highly reactive Ag(I)-catalyzed [3 + 2] cycloaddition of azomethine ylides is founded using AgOAc as the catalytic precursor and phosphines as ligands. Using a new bis-ferrocenyl amide phosphine (FAP) as the ligand, we found that high enantioselectivities (up to 97% ee) have been achieved in the [3 + 2] cycloaddition of azomethine ylides. Up to four stereogenic centers can be established in this multicomponent coupling reaction from readily available materials such as aldehydes, aminoesters, and dipolarophiles.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
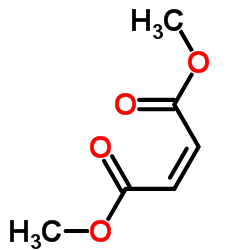 |
顺丁烯二甲酸二甲酯
CAS:624-48-6 |
C6H8O4 |
相关文献:
更多...
|
Fumarate analogs act as allosteric inhibitors of the human m...
2014-01-01 [PLoS ONE 9(6) , e98385, (2014)] |
|
Sensitization to dimethyl fumarate with multiple concurrent ...
2010-02-01 [Contact Dermatitis 62(2) , 88-96, (2010)] |
|
Use of p-nitrophenyl disulfide to measure reductive capacity...
1995-01-01 [Meth. Enzymol. 251 , 279-86, (1995)] |
|
Maleic acid dimethylester: evaluation of dermal toxicity and...
1991-08-01 [Food Chem. Toxicol. 29(8) , 575-8, (1991)] |
|
Experimental and computational evidence for alpha-lactone in...
2007-12-21 [Org. Biomol. Chem. 5(24) , 4001-9, (2007)] |