Esterification of 9-fluorenylmethoxycarbonyl-glycosylated serine and cysteine derivatives with an hydroxymethyl resin.
E Harth-Fritschy, D Cantacuzène
文献索引:J. Pept. Res. 50(6) , 415-20, (1997)
全文:HTML全文
摘要
Esterification of glycosylated serine and cysteine derivatives with a 4-alkoxybenzyl alcohol (Wang) resin is described. The classical methods of ester bond formation (symmetrical anhydride, 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate [TBTU]/4-dimethylaminopyridine [DMAP] with or without 1-hydroxybenzotriazole [HOBT], pentafluorophenyl [Pfp] esters gave high percentages of racemization of the glycosylated serine or cysteine residues. To reduce the D-amino acid content, we found that the best results were obtained with the highly efficient MSNT reagent (2,4,6-mesitylenesulfonyl-3-nitro-1,2,4-triazolide), which gave a high yield of substitution of the resin and the lowest percentage of racemization. A difference in behavior was observed between the two amino acids. The glycosylated cysteine derivative always gave lower racemization than the analogous glycosylated serine.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
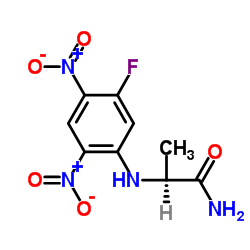 |
N-α-(2,4-二硝基-5-氟苯基)-L-丙氨酸
CAS:95713-52-3 |
C9H9FN4O5 |
|
Structural analysis reveals the substrate-binding mechanism ...
2015-04-13 [ChemBioChem. 16(6) , 924-9, (2015)] |
|
Biosynthesis of the new broad-spectrum lipopeptide antibioti...
2014-04-01 [Res. Microbiol. 165(3) , 243-51, (2014)] |
|
Structure elucidation and biosynthesis of fuscachelins, pept...
2008-10-07 [Proc. Natl. Acad. Sci. U. S. A. 105 , 15311-6, (2008)] |
|
Use of Marfey's reagent and analogs for chiral amino acid an...
2011-11-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 879(29) , 3148-61, (2011)] |
|
Chromatographic separation of enantiomers of non-protein alp...
2009-03-01 [Amino Acids 36(3) , 571-9, (2009)] |