Chromatographic separation of enantiomers of non-protein alpha-amino acids after derivatization with Marfey's reagent and its four variants.
R Bhushan, Virender Kumar, Shivani Tanwar
文献索引:Amino Acids 36(3) , 571-9, (2009)
全文:HTML全文
摘要
Some non-protein alpha-amino acids were derivatized with 1-fluoro-2,4-dinitrophenyl-5-L-alaninamide (Marfey's reagent, MR, FDNP-L-Ala-NH(2),) and four of its structural variants FDNP-L-Phe-NH(2), FDNP-L-Val-NH(2), FDNP-L-Leu-NH(2) and FDNP-L-Pro-NH(2). The resultant diastereomers were separated by normal and reversed phase thin layer chromatography (TLC) and reversed phase HPLC. In normal phase TLC, best resolution was obtained with solvent combination of phenol-water (3:1) while in reversed phase TLC mixtures of acetonitrile with triethylammonium phosphate buffer were found successful for resolution of diastereomers. The separation behavior of diastereomers prepared with different reagents was compared. The diastereomers of most of the amino acids prepared with FDNP-L-Leu-NH(2) were best separated while those prepared with FDNP-L-Pro-NH(2) failed to separate in most of the cases. The diastereomers were also separated on a reversed phase C(8) column with gradient elution using mixture of aqueous-trifluoroacetic acid (TFA) and acetonitrile and with detection at 340 nm. The effects of TFA concentration, flow rate and run time on HPLC separation were studied.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
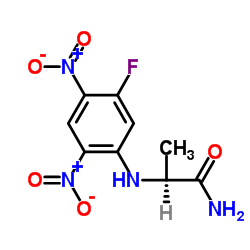 |
N-α-(2,4-二硝基-5-氟苯基)-L-丙氨酸
CAS:95713-52-3 |
C9H9FN4O5 |
|
Structural analysis reveals the substrate-binding mechanism ...
2015-04-13 [ChemBioChem. 16(6) , 924-9, (2015)] |
|
Biosynthesis of the new broad-spectrum lipopeptide antibioti...
2014-04-01 [Res. Microbiol. 165(3) , 243-51, (2014)] |
|
Structure elucidation and biosynthesis of fuscachelins, pept...
2008-10-07 [Proc. Natl. Acad. Sci. U. S. A. 105 , 15311-6, (2008)] |
|
Use of Marfey's reagent and analogs for chiral amino acid an...
2011-11-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 879(29) , 3148-61, (2011)] |
|
Esterification of 9-fluorenylmethoxycarbonyl-glycosylated se...
1997-12-01 [J. Pept. Res. 50(6) , 415-20, (1997)] |