X-Ray structure of the antibiotic bacitracin A.
S Pfeffer, W Höhne, S Branner, K Wilson, C Betzel
文献索引:FEBS Lett. 285(1) , 115-9, (1991)
全文:HTML全文
摘要
Bacitracins are a group of widely used peptide antibiotics. There has been interest in determining the three-dimensional structure of the bacitracins. However, solution studies indicate significant flexibility in their structure and to date native bacitracins have resisted attempts at crystallisation despite considerable efforts over a number of years by several groups. Here we report the first three-dimensional X-ray structure of a bacitracin, complexed to a subtilisin proteinase. X-Ray diffraction data were collected using synchrotron radiation in combination with the Image Plate Scanner system. The complex structure including two enzymes, two bacitracins, 220 water molecules and two Ca2+ ions was refined by restrained least-squares to a crystallographic R factor ( = sigma [[Fo-Fc]]/sigma [Fo]]) of 16.3% at 2.0 A.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
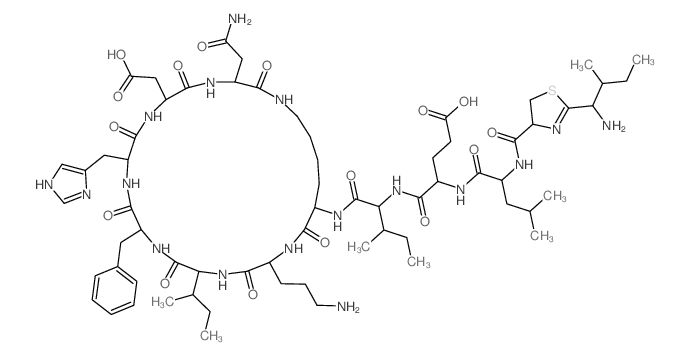 |
杆菌肽 A
CAS:22601-59-8 |
C66H103N17O16S |
|
Validation of a quantitative HPLC method for bacitracin and ...
2012-11-01 [J. Pharm. Biomed. Anal. 70 , 619-23, (2012)] |
|
Dereplicating nonribosomal peptides using an informatic sear...
2012-11-20 [Proc. Natl. Acad. Sci. U. S. A. 109(47) , 19196-201, (2012)] |
|
Affinity capillary electrophoresis study of the linkage exis...
2003-03-01 [Electrophoresis 24(5) , 801-7, (2003)] |
|
Detection of residual bacitracin A, colistin A, and colistin...
2006-05-01 [Anal. Bioanal. Chem 385(1) , 181-8, (2006)] |
|
Total structures and antimicrobial activity of bacitracin mi...
1995-03-01 [J. Antibiot. 48(3) , 233-42, (1995)] |