Affinity capillary electrophoresis study of the linkage existing between proton and zinc ion binding to bacitracin A1.
Massimo Castagnola, Diana Valeria Rossetti, Rosanna Inzitari, Alberto Vitali, Alessandro Lupi, Cecilia Zuppi, Tiziana Cabras, Maria Benedetta Fadda, Raffaele Petruzzelli, Bruno Giardina, Irene Messana
文献索引:Electrophoresis 24(5) , 801-7, (2003)
全文:HTML全文
摘要
Measurements by capillary electrophoresis (CE) of bacitracin A(1) effective mobility at different pH values permitted to estimate the five acidic dissociation constants and the Stokes radii at different protonation stages of the macrocyclic dodecapeptide. The pK(a) values were 3.6 and 4.4 for the two carboxylic groups of the lateral chains of D-Asp-11 and D-Glu-4, respectively, 6.4 for the aza-atom of the imidazole ring of His-10, 7.6 for the amino group of N-terminal Ile-1 and 9.7 for the delta-amino group of D-Orn-7, very close to the values obtained by other researchers by titration experiments. In agreement with a rigid macrocyclic structure the Stokes radii of different protonated forms ranged only between 14.3 and 14.8 A. Best fitting procedures performed on experimental mobility measured at two different pH values (5.50 and 6.72) in the presence of increasing Zn(+2) concentration allowed confirming the model that assumes the binding of Zn(+2) to P(0) peptide form with a 1.5 x 10(3) M(-1) intrinsic association constant. Following to Zn(+2) binding, the pK(a) of the amino group of N-terminal Ile-1 is shifted from 7.6 to 5.9 and the Stokes radius is reduced of about 3 A. The mean charge of the bacitracin A(1)-Zn(+2) complex resulted +1.67 and +1.12 at pH 5.50 and 6.72, respectively. These results suggest that the amino group of N-terminal Ile-1 is not essential for Zn(+2) binding.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
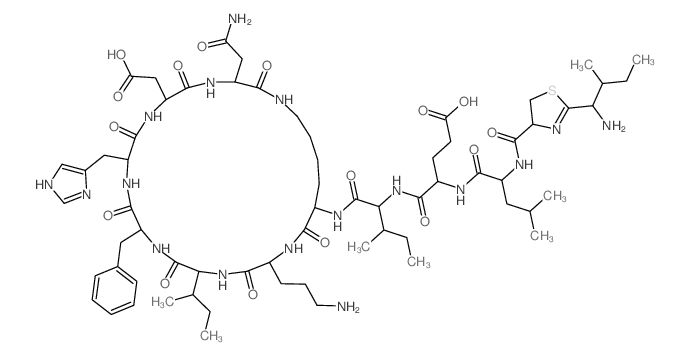 |
杆菌肽 A
CAS:22601-59-8 |
C66H103N17O16S |
|
Validation of a quantitative HPLC method for bacitracin and ...
2012-11-01 [J. Pharm. Biomed. Anal. 70 , 619-23, (2012)] |
|
Dereplicating nonribosomal peptides using an informatic sear...
2012-11-20 [Proc. Natl. Acad. Sci. U. S. A. 109(47) , 19196-201, (2012)] |
|
Detection of residual bacitracin A, colistin A, and colistin...
2006-05-01 [Anal. Bioanal. Chem 385(1) , 181-8, (2006)] |
|
Total structures and antimicrobial activity of bacitracin mi...
1995-03-01 [J. Antibiot. 48(3) , 233-42, (1995)] |
|
1H NMR study on the conformation of bacitracin A in aqueous ...
1992-06-29 [FEBS Lett. 305(2) , 105-9, (1992)] |