| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
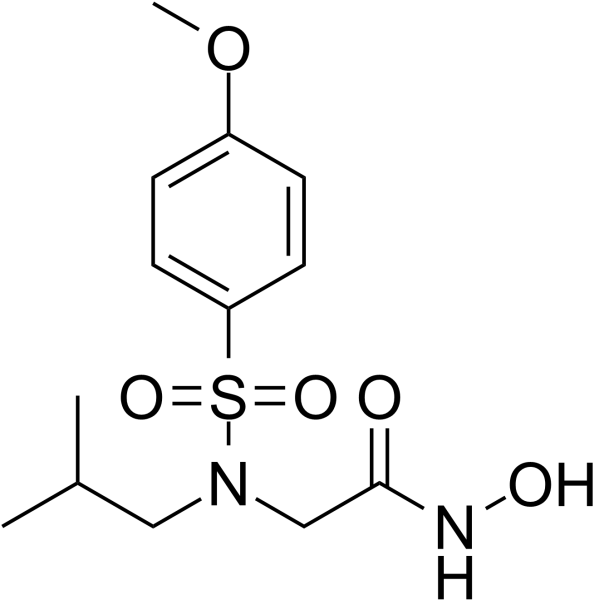 |
NNGH
CAS:161314-17-6 |
|
 |
乙二胺四乙酸
CAS:60-00-4 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
NNGH
CAS:161314-17-6 |
|
 |
乙二胺四乙酸
CAS:60-00-4 |