| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
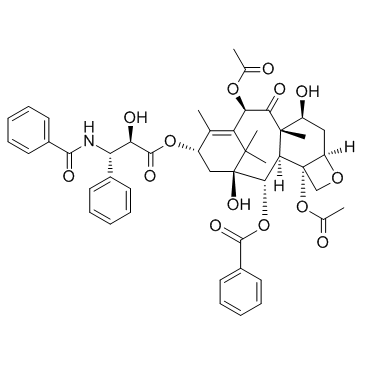 |
紫杉醇
CAS:33069-62-4 |
|
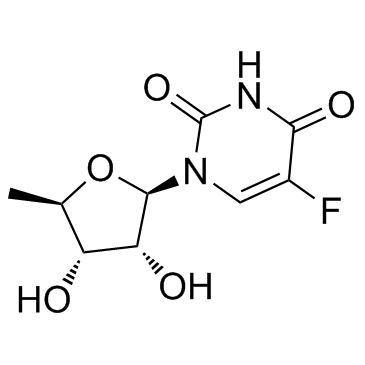 |
去氧氟尿苷
CAS:3094-09-5 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
紫杉醇
CAS:33069-62-4 |
|
 |
去氧氟尿苷
CAS:3094-09-5 |