| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
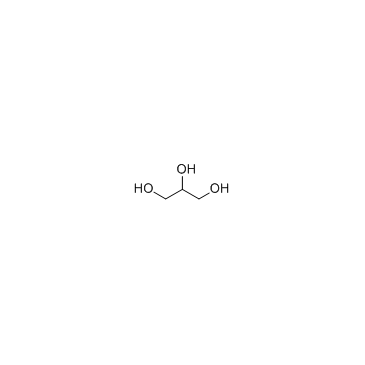 |
甘油
CAS:56-81-5 |
|
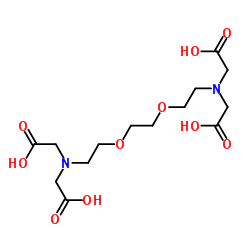 |
3,6-二氧杂-1,8-辛二胺四乙酸(EGTA)
CAS:67-42-5 |
|
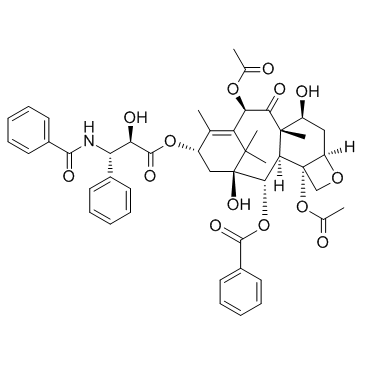 |
紫杉醇
CAS:33069-62-4 |
|
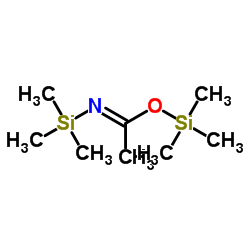 |
N,O-双(三甲基硅基)乙酰胺
CAS:10416-59-8 |