| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
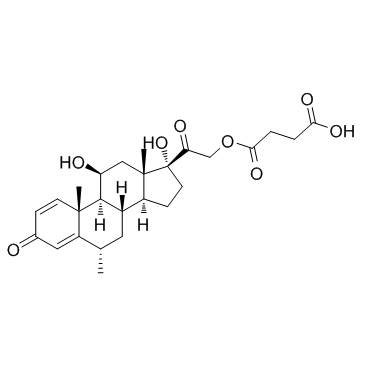 |
甲基泼尼松龙琥珀酸酯
CAS:2921-57-5 |
|
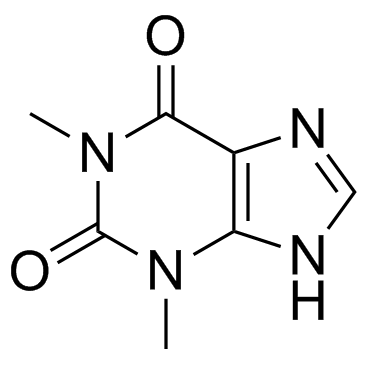 |
茶碱
CAS:58-55-9 |
|
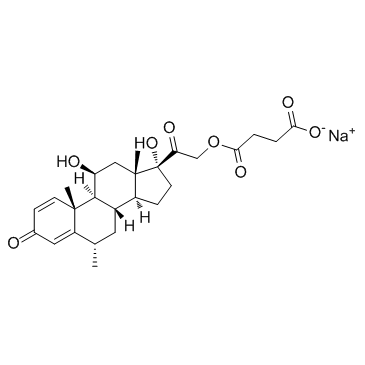 |
甲基泼尼松龙琥珀酸钠
CAS:2375-03-3 |