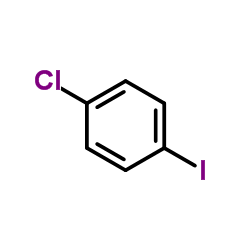
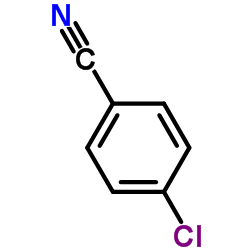
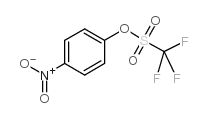
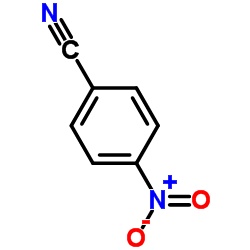
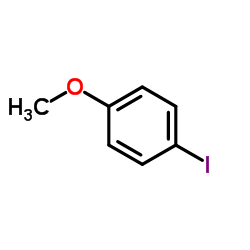
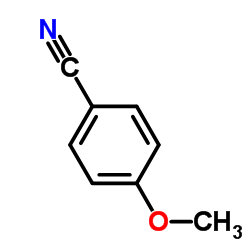
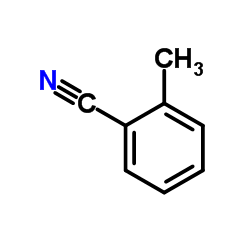
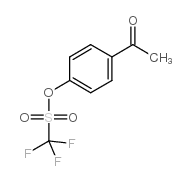
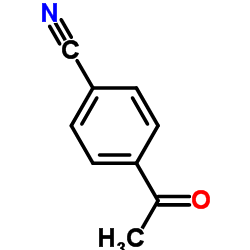
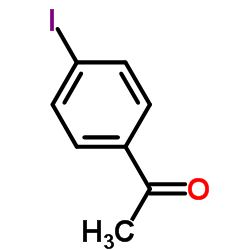
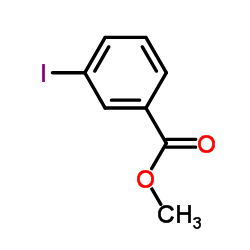
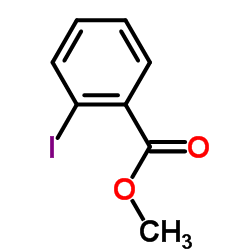
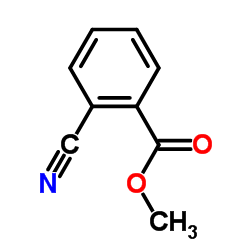
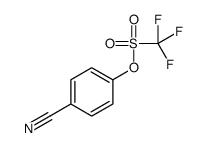
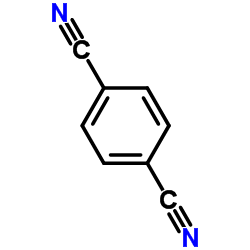
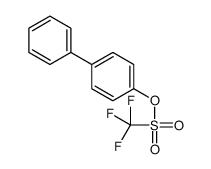
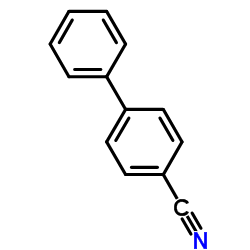
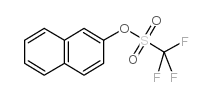
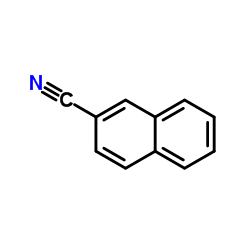
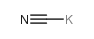|
~93% |
|
~92% |
|
~91% |
|
~87% |
|
~93% |
|
~93% |
|
~95% |
|
~90% |
|
~87% |
|
~93% |
|
~89% |
|
~90% |