Functional properties and biodistribution of poly(triethylenetetramine/cystamine bisacrylamide) and poly(triethylenetetramine/cystamine bisacrylamide)- poly(ethylene glycol) mixtures formed with nucleic acid.
Jonathan H Brumbach, Yong Won Lee, Sung Wan Kim, James W Yockman
文献索引:J. Control. Release 159(1) , 111-9, (2012)
全文:HTML全文
摘要
The clinical success of non-viral gene delivery reagents is hampered by their inefficient cellular transgene delivery, which is largely influenced by carrier properties that are currently undefined and misunderstood. In an attempt to further define and understand the requirements for a safe and efficient non-viral gene delivery reagent, research labs often engineer and evaluate many putative products with subtle physiochemical differences in order to delineate requirements for improved in vitro and in vivo success. The synthesis of many putative reagents is often time-intensive, laborious and costly. In a previous manuscript published by our lab, different amounts of poly(triethylenetetramine/cystamine bisacrylamide) (p(TETA/CBA) and its pegylated counterpart, poly(triethylenetetramine/cystamine bisacrylamide)- poly(ethylene glycol) (p(TETA/CBA)-g-PEG) were mixed together to easily identify optimal reagent properties and candidates in vitro, while avoiding the synthesis of many putative candidates for study. This report uses the aforementioned facile approach to evaluate reagent properties of products that were obtained via one-pot synthesis, which improved synthetic ease. As such, synthesis time was reduced from 6days to 3days and had comparable or improved transfection and viability compared to previous works. Moreover, this synthesis resulted in higher molecular weight products than were used in the previous study and allow for lower polymer doses to be used for complexation, which is useful for systemic delivery that is used herein. The physiochemical properties of the formulations derived using these novel reagents was studied prior to investigating their in vivo biodistribution profiles in a murine colon adenocarcinoma model. Interestingly, negatively charged complexes exhibited greater passive tumor accumulation compared to positively charged complexes following their systemic administration. These studies warrant further investigation for the use of negatively charged drug and gene delivery reagents for passive tumor targeting, and they substantiate the use of polycation/PEG-polycation mixtures for facile product evaluation in order to elucidate design and formulation mandates for the clinical success of non-viral gene delivery formulations.Copyright © 2012 Elsevier B.V. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
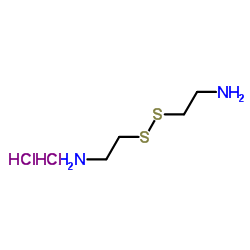 |
胱胺二盐酸盐
CAS:56-17-7 |
C4H14Cl2N2S2 | |
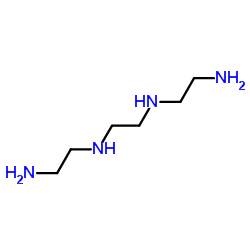 |
三乙烯四胺
CAS:112-24-3 |
C6H18N4 |
|
Curcumin delivery from poly(acrylic acid-co-methyl methacryl...
2015-01-01 [Int. J. Pharm. 486 , 259-67, (2015)] |
|
ROP and ATRP Fabricated Dual Targeted Redox Sensitive Polyme...
2015-05-06 [ACS Appl. Mater. Interfaces 7 , 9211-27, (2015)] |
|
Glycopolymer micelles with reducible ionic cores for hepatoc...
2013-01-30 [Int. J. Pharm. 441(1-2) , 170-80, (2013)] |
|
Gold nanoparticles immobilized hydrophilic monoliths with va...
2015-11-05 [Anal. Chim. Acta 900 , 83-9, (2015)] |
|
Cys-pair reporters detect a constrained trigger loop in a pa...
2013-06-27 [Mol. Cell. 50(6) , 882-93, (2013)] |