| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
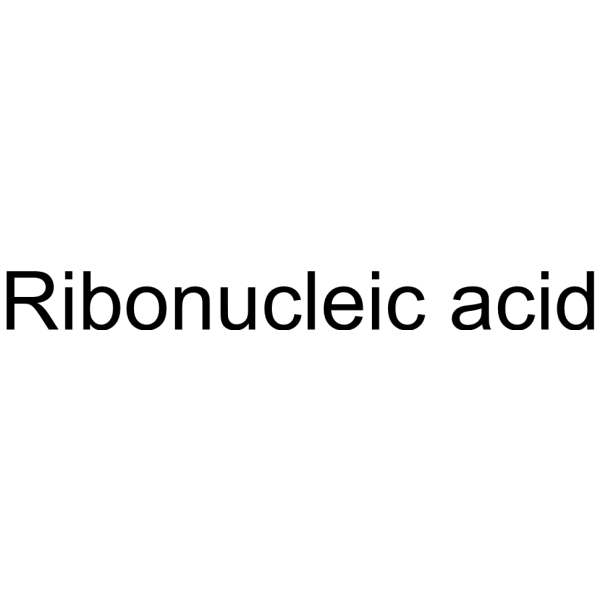 |
核糖核酸 来源于面包酵母(酿酒酵母)
CAS:63231-63-0 |
|
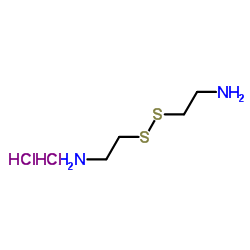 |
胱胺二盐酸盐
CAS:56-17-7 |
|
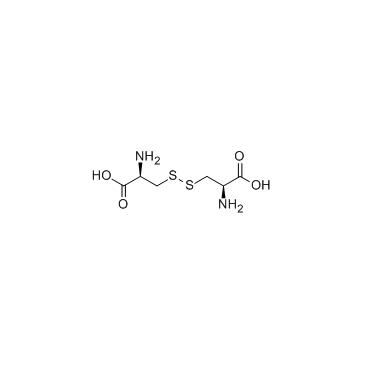 |
L-胱氨酸
CAS:56-89-3 |