| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
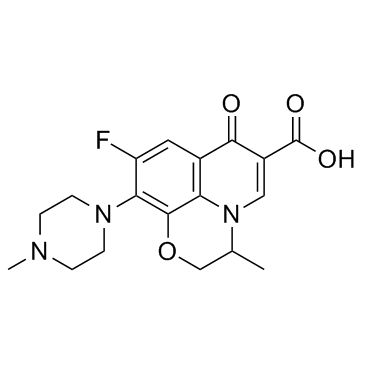 |
氧氟沙星
CAS:82419-36-1 |
|
 |
尼群地平
CAS:39562-70-4 |
|
 |
4-氨基吡啶
CAS:504-24-5 |
|
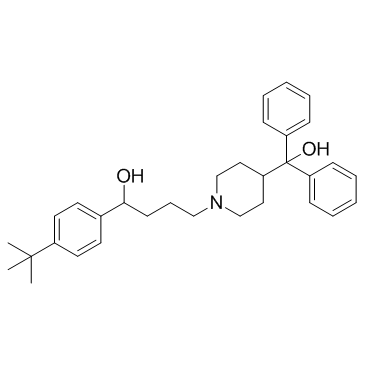 |
特非那定
CAS:50679-08-8 |
|
 |
咖啡因
CAS:58-08-2 |
|
 |
甲氧苄氨嘧啶
CAS:738-70-5 |
|
 |
克霉唑
CAS:23593-75-1 |
|
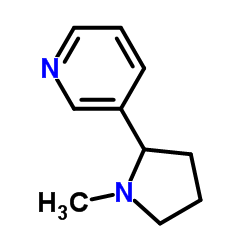 |
(+/-)-尼古丁
CAS:22083-74-5 |
|
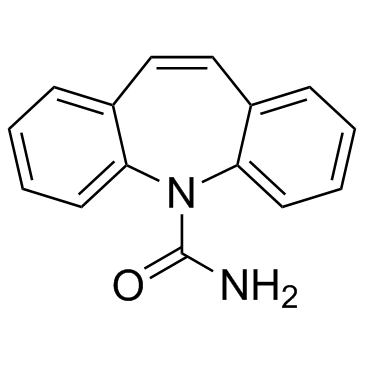 |
卡马西平
CAS:298-46-4 |
|
 |
他莫昔芬
CAS:10540-29-1 |