| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
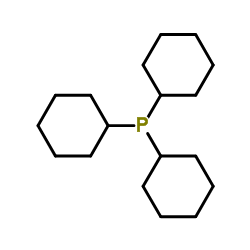 |
三环己基膦
CAS:2622-14-2 |
|
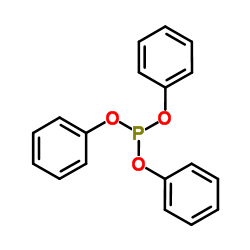 |
亚磷酸三苯酯
CAS:101-02-0 |
|
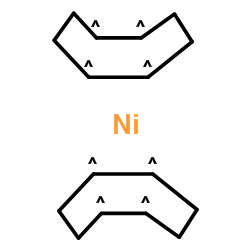 |
双(1,5-环辛二烯)镍(0)
CAS:1295-35-8 |
|
 |
argon-40
CAS:1290046-39-7 |