| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
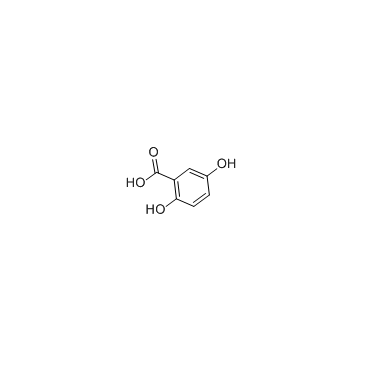 |
2,5-二羟基苯甲酸
CAS:490-79-9 |
|
 |
丙酮
CAS:67-64-1 |
|
 |
N,N-二甲基甲酰胺
CAS:68-12-2 |
|
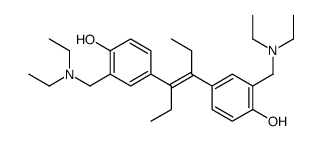 |
NSC 33994
CAS:82058-16-0 |
|
 |
磷酸氢二铵
CAS:7783-28-0 |