N,N-二甲基甲酰胺
一般危化品

N,N-二甲基甲酰胺结构式
|
常用名 | N,N-二甲基甲酰胺 | 英文名 | N,N-Dimethylformamide |
|---|---|---|---|---|
| CAS号 | 68-12-2 | 分子量 | 73.09380 | |
| 密度 | 0.948 g/mL at 20 °C | 沸点 | 153 °C(lit.) | |
| 分子式 | C3H7NO | 熔点 | -61 °C | |
| MSDS | 中文版 美版 | 闪点 | 136 °F | |
| 符号 |



GHS02, GHS07, GHS08 |
信号词 | Danger |
N,N-二甲基甲酰胺用途N、 N-二甲基甲酰胺(DMF)被广泛用作药物溶剂。N、 N-二甲基甲酰胺是一种具有物理化学性质的偶极亲原溶剂,因此适合作为毛细管电泳(CE)的溶剂[1][2]。 |
| 中文名 | N,N-二甲基甲酰胺 |
|---|---|
| 英文名 | N,N-dimethylformamide |
| 中文别名 | 二甲基甲酰胺 |
| 英文别名 | 更多 |
| 描述 | N、 N-二甲基甲酰胺(DMF)被广泛用作药物溶剂。N、 N-二甲基甲酰胺是一种具有物理化学性质的偶极亲原溶剂,因此适合作为毛细管电泳(CE)的溶剂[1][2]。 |
|---|---|
| 相关类别 | |
| 体内研究 | N、 N-二甲基甲酰胺(DMF;N-甲酰二甲胺)具有肝毒性[3]。 |
| 参考文献 |
| 密度 | 0.948 g/mL at 20 °C |
|---|---|
| 沸点 | 153 °C(lit.) |
| 熔点 | -61 °C |
| 分子式 | C3H7NO |
| 分子量 | 73.09380 |
| 闪点 | 136 °F |
| 精确质量 | 73.05280 |
| PSA | 20.31000 |
| LogP | 0.34030 |
| InChIKey | ZMXDDKWLCZADIW-UHFFFAOYSA-N |
| SMILES | CN(C)C=O |
| 外观性状 | 透明无色液体 |
| 蒸汽密度 | 2.5 (vs air) |
| 折射率 | n20/D 1.430(lit.) |
| 储存条件 | 储存注意事项 储存于阴凉、通风的库房。库温不宜超过37℃。远离火种、热源。保持容器密封。应与氧化剂、还原剂、卤素等分开存放,切忌混储。采用防爆型照明、通风设施。禁止使用易产生火花的机械设备和工具。储区应备有泄漏应急处理设备和合适的收容材料。 |
| 稳定性 | 1.为非质子型极性溶剂,对多种有机化合物和无机化合物均有良好的溶解能力,在无碱、酸、水存在下,具有良好的化学稳定性。 2.化学性质:在无酸、碱、水存在下,即使加热到沸点也是比较稳定的。在酸的作用下分解成甲酸和二甲胺盐,而在碱的作用下则分解成甲酸盐和二甲胺。 3.受紫外线作用分解成二甲胺与甲醛,加热到350℃左右分解成二甲胺与一氧化碳。与盐酸形成比较稳定的等摩尔的加合物,其熔点为40℃,沸点为110℃。与SO3也能形成结晶性加合物,其熔点为138℃,沸点为145℃,DMF-SO3可作为缓和的磺化剂和硫酸化剂使用。与POCl3、COCl2、SOCl2等形成的加合物可在电子密度高的芳香环上引入CHO基(Vilsmeier反应)。P2O5在室温下不溶于N,N-二甲基甲酰胺,但在40℃以上形成稳定的络合物后,在室温即能溶解,而不发生沉淀。在金属钠存在下加热时发生激烈反应并放出氢气。与三乙基铝在0℃也能发生激烈反应。也能与Grignard试剂反应。与酰氯及酸酐发生反应时生成二甲酰胺的衍生物。 4.属低毒类。动物试验证明,连续投给大量的N,N-二甲基甲酰胺时,引起体重减轻,并阻碍造血机能。对眼、皮肤、黏膜有强烈的刺激作用,其液体或蒸气被皮肤吸收后还能引起肝脏障碍。吸入高浓度的蒸气能引起急性中毒,主要症状为严重刺激、全身痉挛、疼痛性便秘和恶心、呕吐等。慢性中毒除有皮肤、黏膜刺激外,尚有恶心、呕吐、胸闷、头痛、全身不适、食欲减少、胃痛、便秘、肝大和肝功能变化、尿胆素原和尿胆素亦可增加。使用时要求平均蒸气浓度在29.9mg/m3以下,59.8mg/m3时即出现中毒症状(伤害中枢神经)。大鼠和小鼠的经口毒性LD50为3000~7000mg/kg。嗅觉阈浓度0.14mg/m3,TJ 36-79规定车间空气中最高容许浓度为10mg/m3。 5.稳定性 稳定 6.禁配物 强氧化剂、酰基氯、氯仿、强还原剂、卤素、氯代烃、浓硫酸、发烟硝酸 7.聚合危害 不聚合 |
| 水溶解性 | soluble |
| 分子结构 | 1、摩尔折射率:19.85 2、摩尔体积(cm3/mol):82.6 3、等张比容(90.2K):186 4、表面张力(dyne/cm):25.7 5、介电常数(F/m):36.7 6、偶极距(D):3.86(1D=3.33×10-30C·m) 7、极化率(10-24cm3):7.87 |
| 计算化学 | 1、疏水参数计算参考值(XlogP):-1 2、氢键供体数量:0 3、氢键受体数量:1 4、可旋转化学键数量:0 5、拓扑分子极性表面积(TPSA):20.3 6、重原子数量:5 7、表面电荷:0 8、复杂度:33.9 9、同位素原子数量:0 10、确定原子立构中心数量:0 11、不确定原子立构中心数量:0 12、确定化学键立构中心数量:0 13、不确定化学键立构中心数量:0 14、共价键单元数量:1 |
| 更多 | 1.性状:无色透明或淡黄色液体,有鱼腥味。 2.熔点(℃):-61 3.沸点(℃):153 4.相对密度(水=1):0.95 5.相对蒸气密度(空气=1):2.51 6.饱和蒸气压(kPa):0.5(25℃) 7.燃烧热(kJ/mol):-1921 8.临界温度(℃):374 9.临界压力(MPa):4.48 10.辛醇/水分配系数:-0.87 11.闪点(℃):58(OC) 12.引燃温度(℃):445 13.爆炸上限(%):15.2 14.爆炸下限(%):2.2 15.溶解性:与水混溶,可混溶于多数有机溶剂。 16.折射率(25ºC):1.42817 17.黏度(mPa·s,25ºC):0.802 18.比旋光度(º):0.94 19.燃点(ºC):445 20.蒸发热(KJ/mol,25ºC):47.545 21.蒸发热(KJ/mol,100ºC):43.585 22.蒸发热(KJ/mol,b.p.):38.368 23.熔化热(KJ/mol):16.165 24.燃烧热(KJ/mol):1915.46 25.比热容(KJ/(kg·K),25ºC,定压):2.14 26.电导率(S/m):6×10-8 27.热导率(W/(m·K),20ºC):0.16579 |
2.对环境的影响: 一、健康危害 侵入途径:吸入、食入、经皮吸收。 健康危害:急性中毒:主要有眼和上呼吸道刺激症状、头痛、焦虑、恶心、呕吐、腹痛、便秘等。肝损害一般在中毒数日后出现,肝脏肿大,肝区痛,可出现黄疸。经皮肤吸收中毒者,皮肤出现水泡、水肿、粘糙,局部麻木、瘙痒、灼痛。 慢性影响:有皮肤、粘膜刺激,神经衰弱综合征,血压偏低。尚有恶心、呕吐、胸闷、食欲不振、胃痛、便秘及肝功能变化。 二、毒理学资料及环境行为
毒性:低毒类。 急性毒性:LD 50400mg/kg(大鼠经口);4720mg/kg(兔经皮);LC 509400mg/m 3,2小时(小鼠吸入);人吸入30~60ppm,消化道症状,肝功可异常,有黄疸,尿胆原增加,蛋白尿;人吸入10~20ppm(有时30ppm),头痛,食欲不振,恶心,肝功和心电图正常。 亚急性和慢性毒性:大鼠吸入2500mg/m 3,6小时/天,5天,80%死亡,肝肺有病变;人吸入5.1~49mg/m 3×3年,神衰症候群,血压偏低,肝功能变化。 危险特性:易燃,遇高热、明火或与氧化剂接触,有引起燃烧爆炸的危险。能与浓硫酸、发烟硝酸猛烈反应,甚至发生爆炸。与卤化物(如四氯化碳)能发生剧烈反应。 燃烧(分解)产物:一氧化碳、二氧化碳、氧化氮。 3.现场应急监测方法:气体检测管法 气体速测管(德国德尔格公司产品) 4.实验室监测方法:气相色谱法《作业环境空气中有毒物质检测方法》陈安之主编 色谱/质谱法《水和有害废物的监测分析方法》周文敏等编译 5.环境标准:
6.应急处理处置方法: 一、泄漏应急处理 迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿消防防护服。尽可能切断泄漏源。防止进入下水道、排洪沟等限制性空间。小量泄漏:用砂土或其它不燃材料吸附或吸收。也可以用大量水冲洗,洗水稀释后放入废水系统。大量泄漏:构筑围堤或挖坑收容;用泡沫覆盖,降低蒸气灾害。用防爆泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。 废弃物处置方法:用焚烧法。废料溶于易燃溶剂后,再焚烧。焚烧炉排出的气体要通过碱洗涤器除去有害成分,从纤维沉降槽和聚氯乙烯反应器的洁净溶剂中回收N,N-二甲基甲酰胺。 二、防护措施 呼吸系统防护:空气中浓度超标时,佩戴过滤式防毒面具(半面罩)。 眼睛防护:戴化学安全防护眼镜。 身体防护:穿化学防护服。 手防护:戴橡胶手套。 其它:工作现场严禁吸烟。工作毕,淋浴更衣。 三、急救措施 皮肤接触:脱去被污染的衣着,用大量流动清水冲洗,至少15分钟。就医。 眼睛接触:立即提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15分钟。就医。 吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即进行人工呼吸。就医。 食入:饮足量温水,催吐,就医。 灭火方法:灭火剂:雾状水、抗溶性泡沫、干粉、二氧化碳、砂土。尽可能将容器从火场移至空旷处。喷水保持火场容器冷却,直至灭火结束。 |
|
N,N-二甲基甲酰胺毒理学数据: 1.急性毒性 LD50:4000mg/kg(大鼠经口);4720mg/kg(兔经皮) LC50:9400mg/m3(小鼠吸入,2h) 2.刺激性 家兔经眼:100%,重度刺激(用水冲洗) 3.亚急性与慢性毒性 大鼠吸入2500mg/m3,每天6h,共5d,16只中有8~10只死亡,尸解可见肝脏和肺脏损伤。 N,N-二甲基甲酰胺生态学数据: 1.生态毒性 LC50:1430mg/L(96h)(黑头呆鱼);10000~13000mg/L(96h)(虹鳟鱼) 2.生物降解性 暂无资料 3.非生物降解性 空气中,当羟基自由基浓度为5.00×105个/cm3时,降解半衰期为22h(理论)。 |
| 符号 |



GHS02, GHS07, GHS08 |
|---|---|
| 信号词 | Danger |
| 危害声明 | H226-H312 + H332-H319-H360D |
| 警示性声明 | P201-P280-P305 + P351 + P338-P308 + P313 |
| 个人防护装备 | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| 危害码 (欧洲) | T:Toxic |
| 风险声明 (欧洲) | R20/21;R36;R61 |
| 安全声明 (欧洲) | S53-S45 |
| 危险品运输编码 | UN 2265 3/PG 3 |
| WGK德国 | 1 |
| RTECS号 | LQ2100000 |
| 包装等级 | III |
| 危险类别 | 3 |
| 海关编码 | 2924191000 |
| N,N-二甲基甲酰胺上游产品 10 | |
|---|---|
| N,N-二甲基甲酰胺下游产品 10 | |
自从1899年用甲酸与二甲胺反应首次合成二甲基甲酰胺以后,发展了以不同原料合成二甲基甲酰胺的工艺方法,如二甲胺-一氧化碳法、甲酰胺-二甲胺法、氰氢酸-甲醇法、乙腈-甲醇法、甲酸甲酯-二甲胺法、三氯乙醛-二甲胺法等等。但目前国外的工业化生产仍以二甲胺-一氧化碳法为主。
1、甲酸甲酯-二甲胺法:由甲酸与甲醇酯化生成甲酸甲酯,然后与二甲胺气相反应生成二甲基甲酰胺,再经蒸馏回收甲醇和未反应的甲酸甲酯后进行减压精馏制得成品。
2、二甲胺-一氧化碳法:由二甲胺与一氧化碳在甲醇钠作用下,直接反应而得。反应条件是1.5-2.5MPa和110-150℃。粗品经精馏制得成品。
3、由一氧化碳和甲醇在高压和80-100℃温度下经羰基合成得甲酸甲酯,然后再与二甲胺反应生成二甲基甲酰胺,精馏后得到成品。
4、三氯乙醛法:由三氯乙醛与二甲胺反应而得。
将氯仿与0.52份的三氯乙醛加入反应釜,冷却至30℃以下,通入气态的二甲胺,同时将其佘0.78份的三氯乙醛滴入反应釜,进行反应。反应结束后进行蒸馏操作,当精馏塔塔顶温度为58-64℃时的馏分为氯仿,64-150℃的馏分为氯仿与二甲基甲酰胺的混合物,将此混合液进行减压蒸馏得粗品二甲基甲酰胺,然后将粗晶再进行精馏,即得成品。
 消耗定额(kg/t):二甲胺(40%)2372;三氯乙醛(95%)2543。
消耗定额(kg/t):二甲胺(40%)2372;三氯乙醛(95%)2543。
精制方法:N,N-二甲基甲酰胺常含有水、乙醇、伯胺、仲胺等杂质,并能与2分子水形成HCON(CH3)2·2H2O。要得到高纯度产品,可使用干燥剂与蒸馏并用的方法。首先加入1/10体积的苯,常压下进行共沸蒸馏以除去水。再按下列方法精制:
① 加入无水硫酸镁(25g/L)干燥,减压下2~2.67KPa蒸馏。
② 加入粉状氧化钡,搅动后倾出液体,减压蒸馏。
③ 加入氧化铝粉末(50g/L,500~600℃烧成),混合摇动,减压下(0.67~1.33KPa)蒸馏。
④ 加入三苯基氯硅烷(5~10g/L),120~140℃加热24小时后减压(0.67KPa)蒸馏。
由以上方法所得产品电导率:(1) (0.9~1.5)×10 -7 S/m;(2) (0.4~1.0)×10 -7 S/m;(3) (0.3~0.9)×10 -7 S/m;(4) (0.2~0.5)×10 -7 S/m。5.以工业品二甲基甲酰胺为原料,经提纯而得试剂二甲基甲酰胺。工业品中若含有少量水,可以通过4A分子筛除去。若水分含量较高,可加适量颗粒氢氧化钾,不进摇动并充分静置分层,分去含有甲酸等杂质的水层后,加入二甲基甲酰胺体积1/5的试剂级苯进行常压精馏,当气相温度达130℃时,在残液中加入适量五氧化二磷,加盖振荡3.5h,静置后滤去固体,然后在充氮条件下用氢氧化钾脱水,再在干燥氮气保护下减压精馏,收集中间馏分,即得高纯度产品。
6.二甲胺与二氧化碳在甲醇钠催化下加压合成或二甲胺与甲酸甲酯气相反应制得,也可由二甲胺与三氯乙醛反应而得。
| 海关编码 | 2924191000 |
|---|
|
Acetyltransferase p300/CBP associated Factor (PCAF) regulates crosstalk-dependent acetylation of histone H3 by distal site recognition.
ACS Chem. Biol. 10(1) , 157-64, (2015) Epigenetic regulation is directed, in part, by the correlated placement of histone post-translational modifications, but the mechanisms controlling correlated modifications are incompletely understood... |
|
|
Development of novel formulations to enhance in vivo transdermal permeation of tocopherol.
Acta. Pharm. 64(3) , 299-309, (2014) Tocopherol represents a big challenge for transdermal permeation owing to its extreme hydrophobicity and large molecular mass. The aim of the present study was to develop alpha-tocopherol (T) topical ... |
|
|
Kuwanon V inhibits proliferation, promotes cell survival and increases neurogenesis of neural stem cells.
PLoS ONE 10(2) , e0118188, (2015) Neural stem cells (NSCs) have the ability to proliferate and differentiate into neurons and glia. Regulation of NSC fate by small molecules is important for the generation of a certain type of cell. T... |
| N,N-dimethyl-malonamic acid ethyl ester |
| N,N-DIMETHYLFORMAMIDE |
| N,N-dimethyl-formamide |
| Dimethylformamide |
| MFCD00003284 |
| dimetylformamide |
| DIMETHYL FORMAMIDE |
| EINECS 200-679-5 |
| ethyl a-dimethylcarbamoylacetate |
| Dimethylcarbamoylessigsaeure-ethylester |
| ethyl N,N-dimethylamidomalonate |
| N,N-Dimethylformamid |
| DMF |

 CAS号201230-82-2
CAS号201230-82-2 CAS号124-40-3
CAS号124-40-3 CAS号124-38-9
CAS号124-38-9 CAS号64-18-6
CAS号64-18-6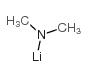 CAS号3585-33-9
CAS号3585-33-9 CAS号506-59-2
CAS号506-59-2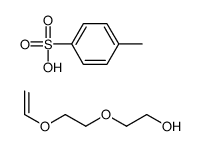 CAS号117731-86-9
CAS号117731-86-9 CAS号107-31-3
CAS号107-31-3 CAS号75-50-3
CAS号75-50-3 CAS号50-00-0
CAS号50-00-0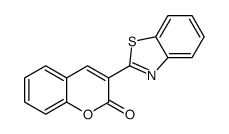 CAS号1032-98-0
CAS号1032-98-0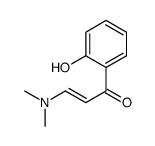 CAS号106129-86-6
CAS号106129-86-6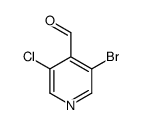 CAS号1064678-66-5
CAS号1064678-66-5 CAS号10045-65-5
CAS号10045-65-5![呋喃并[3,2-c]吡啶-2-羧醛结构式](https://image.chemsrc.com/caspic/074/112372-07-3.png) CAS号112372-07-3
CAS号112372-07-3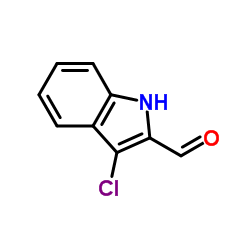 CAS号110912-15-7
CAS号110912-15-7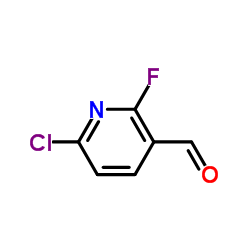 CAS号1093880-37-5
CAS号1093880-37-5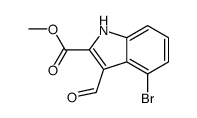 CAS号1079252-75-7
CAS号1079252-75-7![呋喃并[3,2-b]吡啶-2-羧醛结构式](https://image.chemsrc.com/caspic/150/112372-05-1.png) CAS号112372-05-1
CAS号112372-05-1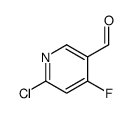 CAS号1060809-20-2
CAS号1060809-20-2

