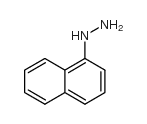
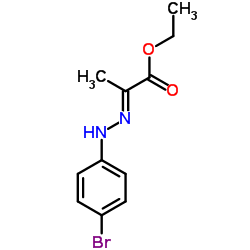
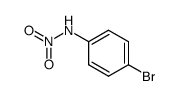
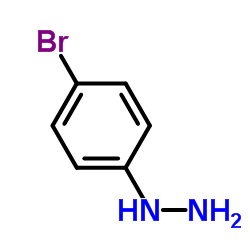
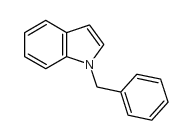
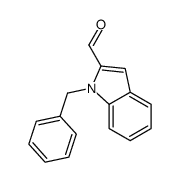
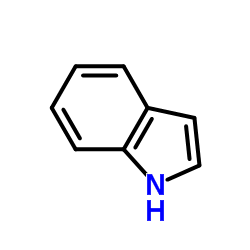
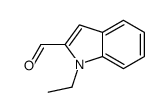
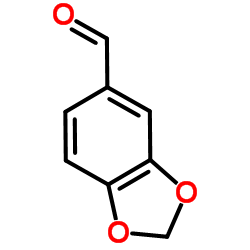
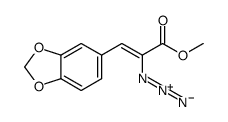
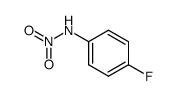
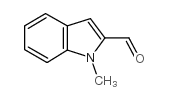
|
~% |
|
~% |
|
~% |
|
~86% |
|
~83% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![ethyl 1H-benzo[g]indole-2-carboxylate结构式](https://image.chemsrc.com/caspic/379/52280-53-2.png)
![[1]naphthyl-nitro-amine结构式](https://image.chemsrc.com/caspic/482/4323-69-7.png)