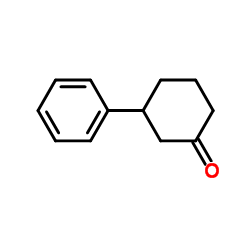
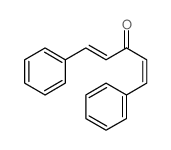
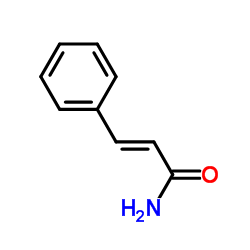
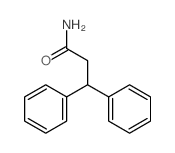
|
~52% |
|
~4% |
|
~7% |
|
~79% |
|
~40% |
|
~21% |
|
~0% |
|
~91% |
|
~23% |
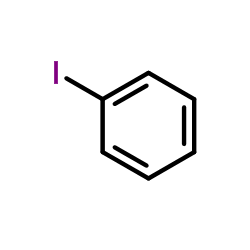
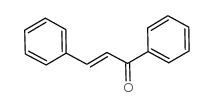

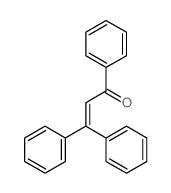
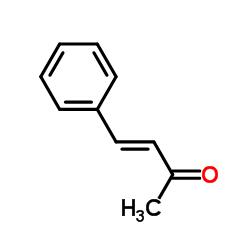
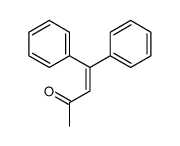
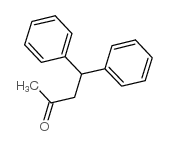
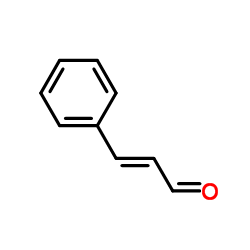
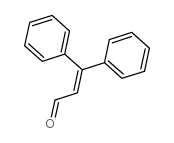
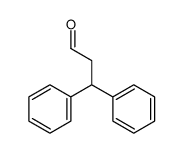
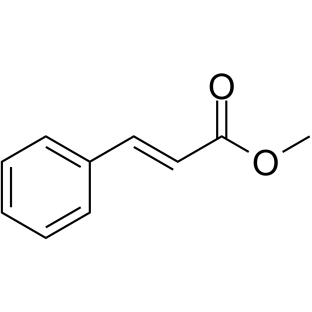

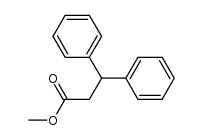
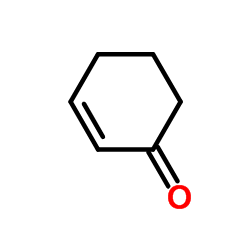
![5,6-二氢[1,1'-联苯]-3(4H)-酮结构式](https://image.chemsrc.com/caspic/412/10345-87-6.png)