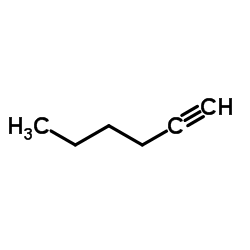
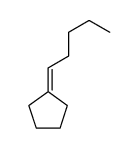
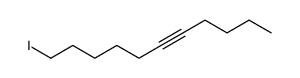
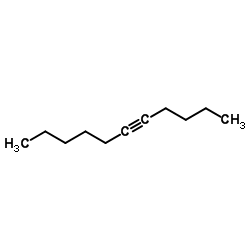
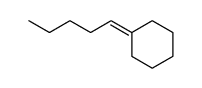
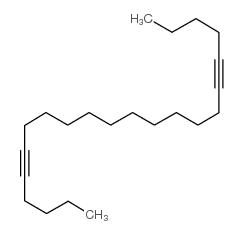
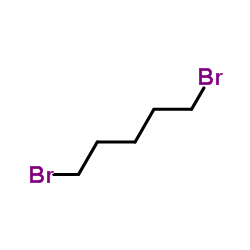
|
~% |
|
~%
详细
|
|
~% |
|
~% |
|
~% |
|
~% |