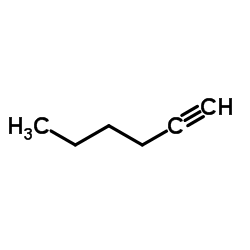
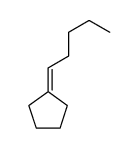
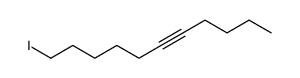
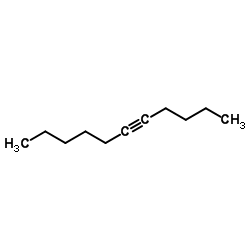
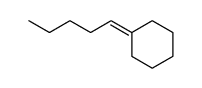
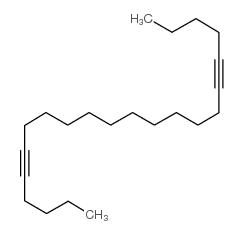
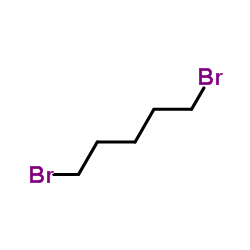
|
~% |
|
~%
Detail
|
|
~% |
|
~% |
|
~% |
|
~% |