Synthesis of NF2Cl and NFCl2 using NH4F/nHF and ClF3
Tatsuo Miyazaki, Isamu Mori, Tomonori Umezaki, Susumu Yonezawa
文献索引:10.1016/j.jfluchem.2018.03.010
全文:HTML全文
摘要
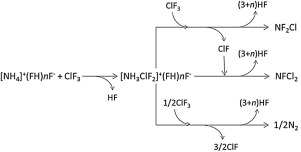
Reactions between NH4F/nHF and gaseous ClF3 were examined at temperatures of 280–320 K under various n-values (n = 2.2, 2.3, 2.4, 2.7, 2.8, 3.0, 3.1, and 4.0) at which NH4F/nHF was a molten salt, with three ClF3 gas flow rates (20, 50 and 100 standard cc min-1). From a direct reaction, NF2Cl and NFCl2 gases were obtained as products. With high NF2Cl and NFCl2 selectivity for N2, such as higher than 90% selectivity as the nitrogen containing molecule base at temperatures below 298 K, an n value of around 2.2–2.3 was obtained. The selectivity of NF2Cl and NFCl2 decreased to less than 70% at temperatures higher than 318 K because of increased amounts of N2 by-product. High selectivity of NF2Cl and NFCl2 were obtainable with the reaction mechanism in which [NH3ClF2]+(FH)nF- is formed as an intermediate.
|
Synthesis and Characterization of Pentafluorosulfanyl-Functi...
2018-04-07 [10.1016/j.jfluchem.2018.04.004] |
|
Theoretical Studies on the Mechanism and Kinetics of the Rea...
2018-03-31 [10.1016/j.jfluchem.2018.03.017] |
|
Characterization of electronic features of intermolecular in...
2018-03-30 [10.1016/j.jfluchem.2018.03.016] |
|
Structure and photoluminescence properties study of neodymiu...
2018-03-30 [10.1016/j.jfluchem.2018.03.015] |
|
Study of Fluoride Content in Some Commercial Phosphate Ferti...
2018-03-29 [10.1016/j.jfluchem.2018.03.018] |