|
~62% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

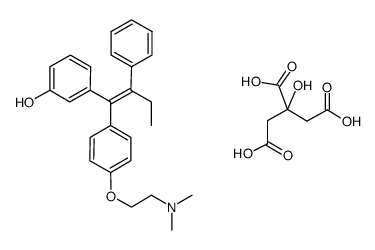
![3-{(Z)-1-[4-(2-DIMETHYLAMINOETHOXY)PHENYL]-2-PHENYLBUT-1-ENYL}PHENOL结构式](https://image.chemsrc.com/caspic/469/83647-29-4.png)



![4'-[2-(N,N-dimethylamino)ethoxy]-3-(trimethylacetoxy)benzophenone结构式](https://image.chemsrc.com/caspic/206/263395-64-8.png)
![4'-[2-(N,N-dimethylamino)ethoxy]-3-hydroxybenzophenone结构式](https://image.chemsrc.com/caspic/168/263395-65-9.png)