|
~60% |
|
~95% |
|
~0% |
|
~92% |
|
~92% |
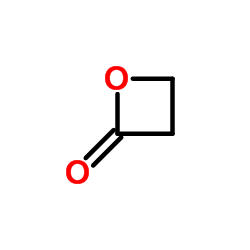
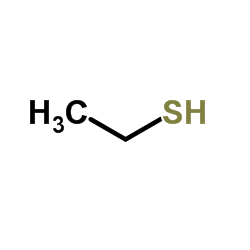
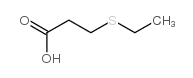
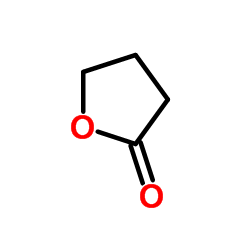

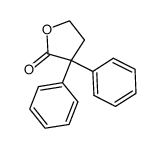

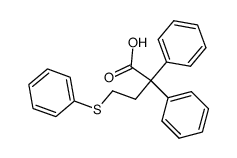



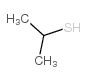
![Butanoic acid,4-[(1-methylethyl)thio]结构式](https://image.chemsrc.com/caspic/297/79313-54-5.png)