K252b
Modify Date: 2025-08-20 08:21:53
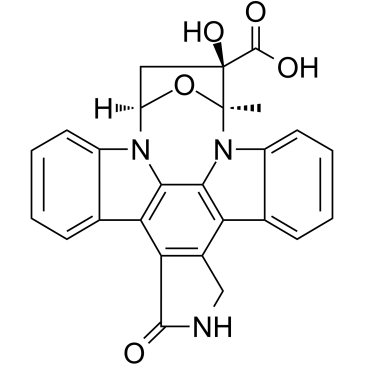
K252b structure
|
Common Name | K252b | ||
|---|---|---|---|---|
| CAS Number | 99570-78-2 | Molecular Weight | 453.446 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 769.8±60.0 °C at 760 mmHg | |
| Molecular Formula | C26H19N3O5 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 419.4±32.9 °C | |
Use of K252bK-252b, an indolocarbazole isolated from the actinomycete Nocardiopsis, is a PKC inhibitor. K-252b can be used to inhibit extracellular kinases of cells in culture because it can’t pass through cell membrane freely [1][2][3]. |
| Name | (5R,6S,8S)-6-hydroxy-5-methyl-13-oxo-5,6,7,8,14,15-hexahydro-13H-5,8-epoxy-4b,8a,14-triazadibenzo[b,h]cycloocta[1,2,3,4-jkl]cyclopenta[e]-as-indacene-6-carboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | K-252b, an indolocarbazole isolated from the actinomycete Nocardiopsis, is a PKC inhibitor. K-252b can be used to inhibit extracellular kinases of cells in culture because it can’t pass through cell membrane freely [1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
PKC[1]. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 769.8±60.0 °C at 760 mmHg |
| Molecular Formula | C26H19N3O5 |
| Molecular Weight | 453.446 |
| Flash Point | 419.4±32.9 °C |
| Exact Mass | 453.132477 |
| PSA | 105.72000 |
| LogP | 3.57 |
| Vapour Pressure | 0.0±2.8 mmHg at 25°C |
| Index of Refraction | 1.903 |
| Hazard Codes | Xi: Irritant; |
|---|---|
| RIDADR | UN 1993 / PGIII |
| Precursor 9 | |
|---|---|
| DownStream 1 | |
|
Cooperativity between extracellular adenosine 5'-triphosphate and activation of N-methyl-D-aspartate receptors in long-term potentiation induction in hippocampal CA1 neurons.
Neuroscience 113 , 617-628, (2002) The mechanism of ATP-induced long-term potentiation (LTP) was studied pharmacologically using guinea-pig hippocampal slices. LTP, induced in CA1 neurons by 10 min application of 10 microM ATP, was blo... |
| 9,12-Epoxy-1H-diindolo[1,2,3-fg:3',2',1'-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylicacid,2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-,(9S,10R,12R) |
| K-252b solution |
| MFCD00132117 |
| (15R,16S,18S)-16-Hydroxy-15-methyl-3-oxo-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1.0.0.0.0.0]octacosa-1,6,8,10,12,20,22,24,26-nonaene-16-carboxylic acid |
| 6,9-Epoxy-15H-diindolo[1,2,3-fg:3',2',1'-kl]pyrrolo[3,4-i][1,6]benzodiazocine-7-carboxylic acid, 6,7,8,9,16,17-hexahydro-7-hydroxy-6-methyl-15-oxo-, (6R,7S,9S)- |
| (5R,6S,8S)-6-hydroxy-5-methyl-13-oxo-5,6,7,8,14,15-hexahydro-13H-5,8-epoxy-4b,8a,14-triazadibenzo[b,h]cycloocta[1,2,3,4-jkl]cyclopenta[e]-as-indacene-6-carboxylic acid |
![(5S,8R)-5-methyl-7,8,14,15-tetrahydro-5H-16-oxa-4b,8a,14-triaza-5,8-methanodibenzo[b,h]cycloocta[jkl]cyclopenta[e]-as-indacene-6,13-dione Structure](https://image.chemsrc.com/caspic/315/111359-05-8.png) CAS#:111359-05-8
CAS#:111359-05-8![13-((2R,4S,5S)-4-hydroxy-5-(iodomethyl)tetrahydrofuran-2-yl)-6,7,12,13-tetrahydro-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazol-5-one Structure](https://image.chemsrc.com/caspic/319/244128-11-8.png) CAS#:244128-11-8
CAS#:244128-11-8![13-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-6,7,12,13-tetrahydro-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazol-5-one Structure](https://image.chemsrc.com/caspic/433/236113-24-9.png) CAS#:236113-24-9
CAS#:236113-24-9![(3S,5R)-2-methylene-5-(7-oxo-6,7-dihydro-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazol-12(13H)-yl)tetrahydrofuran-3-yl acetate Structure](https://image.chemsrc.com/caspic/157/244128-15-2.png) CAS#:244128-15-2
CAS#:244128-15-2![(5S,6S,8R)-5-methyl-13-oxo-6,7,8,13,14,15-hexahydro-5H-16-oxa-4b,8a,14-triaza-5,8-methanodibenzo[b,h]cycloocta[jkl]cyclopenta[e]-as-indacen-6-yl acetate Structure](https://image.chemsrc.com/caspic/081/244128-17-4.png) CAS#:244128-17-4
CAS#:244128-17-4![(5S,6S,8R)-6-cyano-5-methyl-13-oxo-5,6,7,8,14,15-hexahydro-13H-16-oxa-4b,8a,14-triaza-5,8-methanodibenzo[b,h]cycloocta[jkl]cyclopenta[e]-as-indacen-6-yl acetate Structure](https://image.chemsrc.com/caspic/043/244128-18-5.png) CAS#:244128-18-5
CAS#:244128-18-5![(5R,6S,8R)-5-(iodomethyl)-13-oxo-6,7,8,13,14,15-hexahydro-5H-16-oxa-4b,8a,14-triaza-5,8-methanodibenzo[b,h]cycloocta[jkl]cyclopenta[e]-as-indacen-6-yl acetate Structure](https://image.chemsrc.com/caspic/119/244128-16-3.png) CAS#:244128-16-3
CAS#:244128-16-3![13-((2R,4S,5S)-4-hydroxy-5-((phenylselanyl)methyl)tetrahydrofuran-2-yl)-6,7,12,13-tetrahydro-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazol-5-one Structure](https://image.chemsrc.com/caspic/233/244128-13-0.png) CAS#:244128-13-0
CAS#:244128-13-0![(2S,3S,5R)-5-(7-oxo-6,7-dihydro-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazol-12(13H)-yl)-2-((phenylselanyl)methyl)tetrahydrofuran-3-yl acetate Structure](https://image.chemsrc.com/caspic/195/244128-14-1.png) CAS#:244128-14-1
CAS#:244128-14-1 CAS#:99533-80-9
CAS#:99533-80-9