(2S,3S)-1,2-Epoxy-3-(Boc-Amino)-4-Phenylbutane
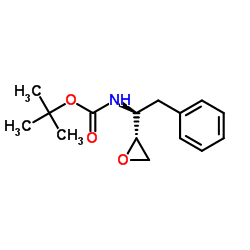
(2S,3S)-1,2-Epoxy-3-(Boc-Amino)-4-Phenylbutane structure
|
Common Name | (2S,3S)-1,2-Epoxy-3-(Boc-Amino)-4-Phenylbutane | ||
|---|---|---|---|---|
| CAS Number | 98737-29-2 | Molecular Weight | 263.332 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 398.8±25.0 °C at 760 mmHg | |
| Molecular Formula | C15H21NO3 | Melting Point | 125-127ºC | |
| MSDS | Chinese USA | Flash Point | 195.0±23.2 °C | |
| Symbol |

GHS09 |
Signal Word | Warning | |
| Name | (2S,3S)-1,2-Epoxy-3-(Boc-Amino)-4-Phenylbutane |
|---|---|
| Synonym | More Synonyms |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 398.8±25.0 °C at 760 mmHg |
| Melting Point | 125-127ºC |
| Molecular Formula | C15H21NO3 |
| Molecular Weight | 263.332 |
| Flash Point | 195.0±23.2 °C |
| Exact Mass | 263.152130 |
| PSA | 50.86000 |
| LogP | 3.44 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.533 |
|
Structure-based design of novel HIV-1 protease inhibitors to combat drug resistance.
J. Med. Chem. 49 , 5252, (2006) Structure-based design and synthesis of novel HIV protease inhibitors are described. The inhibitors are designed specifically to interact with the backbone of HIV protease active site to combat drug r... |
|
|
Discovery of HIV-1 protease inhibitors with picomolar affinities incorporating N-aryl-oxazolidinone-5-carboxamides as novel P2 ligands.
J. Med. Chem. 49 , 7342, (2006) Here, we describe the design, synthesis, and biological evaluation of novel HIV-1 protease inhibitors incorporating N-phenyloxazolidinone-5-carboxamides into the (hydroxyethylamino)sulfonamide scaffol... |
|
|
Probing pockets S2-S4' of the gamma-secretase active site with (hydroxyethyl)urea peptidomimetics.
Bioorg. Med. Chem. Lett. 14 , 1935-1938, (2004) (Hydroxyethyl)urea peptidomimetics are potent inhibitors of gamma-secretase that are accessible in a few synthetic steps. Systematic alteration of P2-P4' revealed that the corresponding S2-S4' active ... |
| Carbamic acid, N-[(1S)-1-(2R)-2-oxiranyl-2-phenylethyl]-, 1,1-dimethylethyl ester |
| tert-Butyl {(1S)-1-[(2R)-oxiran-2-yl]-2-phenylethyl}carbamate |
| (2S,3S)-N-t-Boc-3-amino-1,2-epoxy-4-phenylbutane |
| tert-Butyl-{(1S)-1-[(2R)-oxiran-2-yl]-2-phenylethyl}carbamat |
| 2-Methyl-2-propanyl {(1S)-1-[(2R)-2-oxiranyl]-2-phenylethyl}carbamate |
| Carbamic acid, N-[(1S)-1-[(2R)-oxiranyl]-2-phenylethyl]-, 1,1-dimethylethyl ester |
| T3OTJ BY1R&MVOX1&1&1 &&(1S)-(2R)- Form |
| MFCD02258997 |
| EINECS 425-420-5 |

