| Structure | Name/CAS No. | Articles |
|---|---|---|
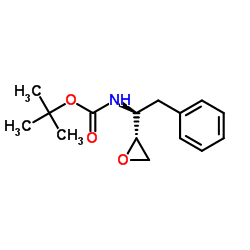 |
(2S,3S)-1,2-Epoxy-3-(Boc-Amino)-4-Phenylbutane
CAS:98737-29-2 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
(2S,3S)-1,2-Epoxy-3-(Boc-Amino)-4-Phenylbutane
CAS:98737-29-2 |