Chloroquinoxaline sulfonamide
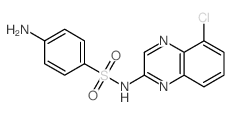
Chloroquinoxaline sulfonamide structure
|
Common Name | Chloroquinoxaline sulfonamide | ||
|---|---|---|---|---|
| CAS Number | 97919-22-7 | Molecular Weight | 334.78100 | |
| Density | 1.569g/cm3 | Boiling Point | 576.3ºC at 760mmHg | |
| Molecular Formula | C14H11ClN4O2S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 302.3ºC | |
Use of Chloroquinoxaline sulfonamideChloroquinoxaline sulfonamide (Chloroquinoxaline), a structural analogue of sulfaquinoxaline, is a topoisomerase II alpha/beta poison. Chloroquinoxaline sulfonamide is used to control coccidiosis in poultry, rabbit, sheep, and cattle[1]. Antitumor activity[2]. |
| Name | 4-amino-N-(5-chloroquinoxalin-2-yl)benzenesulfonamide |
|---|---|
| Synonym | More Synonyms |
| Description | Chloroquinoxaline sulfonamide (Chloroquinoxaline), a structural analogue of sulfaquinoxaline, is a topoisomerase II alpha/beta poison. Chloroquinoxaline sulfonamide is used to control coccidiosis in poultry, rabbit, sheep, and cattle[1]. Antitumor activity[2]. |
|---|---|
| Related Catalog | |
| Target |
topoisomerase II alpha topoisomerase II beta |
| In Vitro | The Chloroquinoxaline sulfonamide IC50 for CV-1cells, obtained using an MTT cytotoxicity assay, was 1.8 mM. Chloroquinoxaline sulfonamide causes dose-dependent protein-DNA cross-links to CV-1 monkey kidney cell chromosomal DNA when drug treatment was terminated by lysis with GuHCl. Chloroquinoxaline sulfonamide-induced protein-DNA cross-links in CV-1 cells. Chloroquinoxaline sulfonamide-induced topoisomerase II-DNA cross-links[1]. Chloroquinoxaline sulfonamide (Chloroquinoxaline), a chlorinated derivative of sulfaquinoxaline, inhibits proliferation of murine B16 melanoma cells, but only when relatively high drug concentrations (1 mM) are used[2]. Cell Proliferation Assay[2] Cell Line: B16 murine melanoma cells Concentration: 10 μM, 100 μM, 1 mM Incubation Time: 24, 48, 72 hours Result: Inhibited proliferation of murine B16 melanoma cells, but only when relatively high drug concentrations (1 mM) were used. |
| References |
| Density | 1.569g/cm3 |
|---|---|
| Boiling Point | 576.3ºC at 760mmHg |
| Molecular Formula | C14H11ClN4O2S |
| Molecular Weight | 334.78100 |
| Flash Point | 302.3ºC |
| Exact Mass | 334.02900 |
| PSA | 106.35000 |
| LogP | 4.40120 |
| Index of Refraction | 1.734 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| HS Code | 2935009090 |
|---|
|
~% 
Chloroquinoxali... CAS#:97919-22-7 |
| Literature: Wolf et al. Journal of the American Chemical Society, 1949 , vol. 71, p. 6,7 |
|
~% 
Chloroquinoxali... CAS#:97919-22-7 |
| Literature: Wolf et al. Journal of the American Chemical Society, 1949 , vol. 71, p. 6,7 |
|
~% 
Chloroquinoxali... CAS#:97919-22-7 |
| Literature: Wolf et al. Journal of the American Chemical Society, 1949 , vol. 71, p. 6,7 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |
| Chlorsulfaquinoxaline |
| chloroquinoxaline sulfonamide |
| Sulfanilsaeure-(5-chlor-chinoxalin-2-ylamid) |
| 4-Amino-N-(5-chloro-2-quinoxalinyl)benzenesulfonamide |
| 5-Chloroquinoxaline-2-sulfanilamide |
| sulfanilic acid-(5-chloro-quinoxalin-2-ylamide) |