Picfeltarraenin IB
Modify Date: 2025-08-24 19:14:25
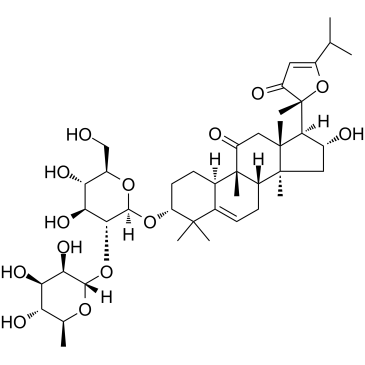
Picfeltarraenin IB structure
|
Common Name | Picfeltarraenin IB | ||
|---|---|---|---|---|
| CAS Number | 97230-46-1 | Molecular Weight | 792.949 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 895.7±65.0 °C at 760 mmHg | |
| Molecular Formula | C42H64O14 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 264.0±27.8 °C | |
Use of Picfeltarraenin IBPicfeltarraenin IB, a triterpenoid obtained from Picriafel-terrae Lour (P.fel-terrae), is an acetylcholinesterase (AChE) inhibitor. Picfeltarraenin IB can be used for the treatment of herpes infections, cancer and inflammation[1]. |
| Name | Picfeltarraenin IB |
|---|---|
| Synonym | More Synonyms |
| Description | Picfeltarraenin IB, a triterpenoid obtained from Picriafel-terrae Lour (P.fel-terrae), is an acetylcholinesterase (AChE) inhibitor. Picfeltarraenin IB can be used for the treatment of herpes infections, cancer and inflammation[1]. |
|---|---|
| Related Catalog | |
| Target |
AChE[1] |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 895.7±65.0 °C at 760 mmHg |
| Molecular Formula | C42H64O14 |
| Molecular Weight | 792.949 |
| Flash Point | 264.0±27.8 °C |
| Exact Mass | 792.429626 |
| PSA | 221.90000 |
| LogP | 5.14 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.607 |
| (1R,4R,9β,16α)-16-Hydroxy-9,10,14-trimethyl-11,22-dioxo-20,24-epoxy-4,9-cyclo-9,10-secocholesta-5,23-dien-1-yl 2-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranoside |
| (1R,4R,9β,16α)-16-Hydroxy-9,10,14-trimethyl-11,22-dioxo-21,24-epoxy-4,9-cyclo-9,10-secocholesta-5,23-dien-1-yl 2-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranoside |
| 19-Norlanosta-5,23-diene-11,22-dione, 3-[[2-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl]oxy]-20,24-epoxy-16-hydroxy-9-methyl-, (3α,9β,10α,16α)- |
| Estr-5-en-11-one, 3-[[2-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl]oxy]-17-[(3R)-3,4-dihydro-6-(1-methylethyl)-4-oxo-2H-pyran-3-yl]-16-hydroxy-4,4,9,14-tetramethyl-, (3α,9β,10alph ; a,16α,17β)- |
| estr-5-en-11-one, 3-[[2-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl]oxy]-17-[(2R)-2,3-dihydro-2-methyl-5-(1-methylethyl)-3-oxo-2-furanyl]-16-hydroxy-4,4,9,14-tetramethyl-, (3α,9β,10α,16α,17β)- |
| Estr-5-en-11-one, 3-[[2-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl]oxy]-17-[(2R)-2,3-dihydro-2-methyl-5-(1-methylethyl)-3-oxo-2-furanyl]-16-hydroxy-4,4,9,14-tetramethyl-, (3α,9β,1 ; 0α,16α,17β)- |